Figure 2.
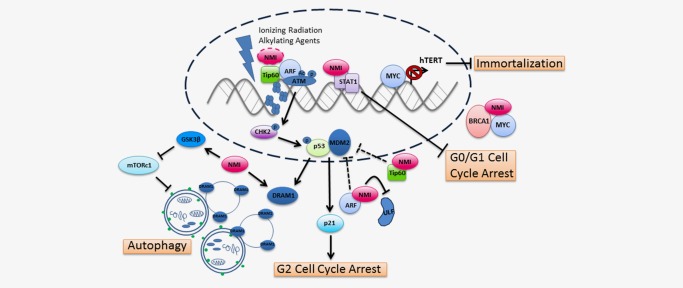
NMI influences cancer initiation. NMI together with BRCA1 sequesters MYC, thereby reducing downstream hTERT gene transcription and inhibiting immortalization of cells during early stages of carcinogenesis. Accumulation of mutations due to DNA damage leads to the transformation of cells. Ionizing radiation and alkylating agents up‐regulate NMI expression and trigger the DNA damage response. Through its interaction with the histone acetyltransferase, Tip60, and tumor suppressor, ARF, NMI could play a role in ATM and p53‐dependent DNA damage responses. The interaction between NMI, ARF and Tip60 may be in a complex to facilitate activation of ATM at sites of DNA damage or independent of each other. Stabilization of ARF by NMI blocks MDM2‐mediated p53 degradation and promotes G2 cell cycle arrest. In addition, NMI may assist Tip60 in its independent inhibition of MDM2 leading to p53 stabilization. However, in contrast, NMI binds STAT1 in glioma cells leading to inhibition of G0/G1 cell cycle arrest. Lastly, NMI facilitates autophagic cell death in response to DNA damage by activating GSK3β and subsequently inhibiting the mTOR pathway—a pathway that blocks autophagy. Expression of DRAM1, a protein essential for the fusing of autophagosomes with lysosomes, positively correlated with NMI levels in breast cancer patients. The dotted connectors indicate possible interactions whereas solid connectors are supported by at least one peer‐reviewed publication.
