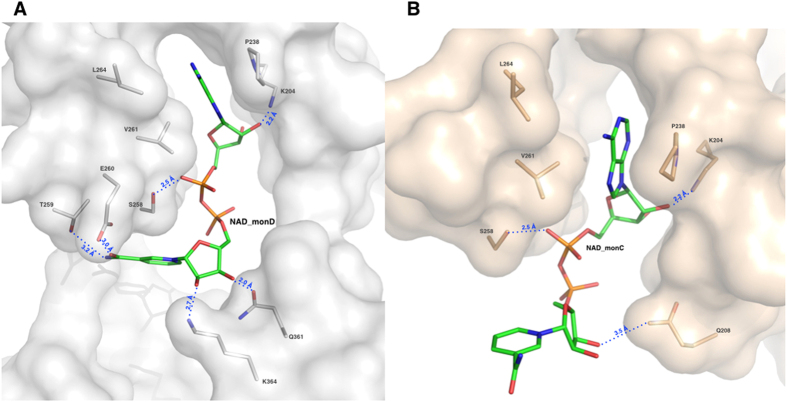
Figure 7. The NAD+ binding site and major interactions established by REA with the protein milieu in its two conformations.
Surface representation of NAD+ binding sites: the monomers D in grey and the monomer C in light-pink. Protein residues are represented as sticks and coloured in light-pink for monomer C and in grey for monomer D; the NAD+ ligands (NAD_C and NAD_D) are shown as sticks and depicted in green. (A) The NAD+ binding mode as observed in monomer D with P238, L264 and V261 contacting the NAD+ adenine moiety and K204 and S258 the adenine ribose. The NAD+ nicotinamide moiety is stabilized through hydrogen bonds established with four amino-acids: K364, Q361, T259 and E260. (B) The NAD+ binding mode as observed in monomer C. The ADP moiety maintains same orientation as in monomer D and establishes same contacts with protein residues. On the contrary, the nicotinamide changes orientation and loses the network of interactions observed in its closed conformation in monomer D and the nicotinamide ribose makes a new contact with Q208.