Figure 2.
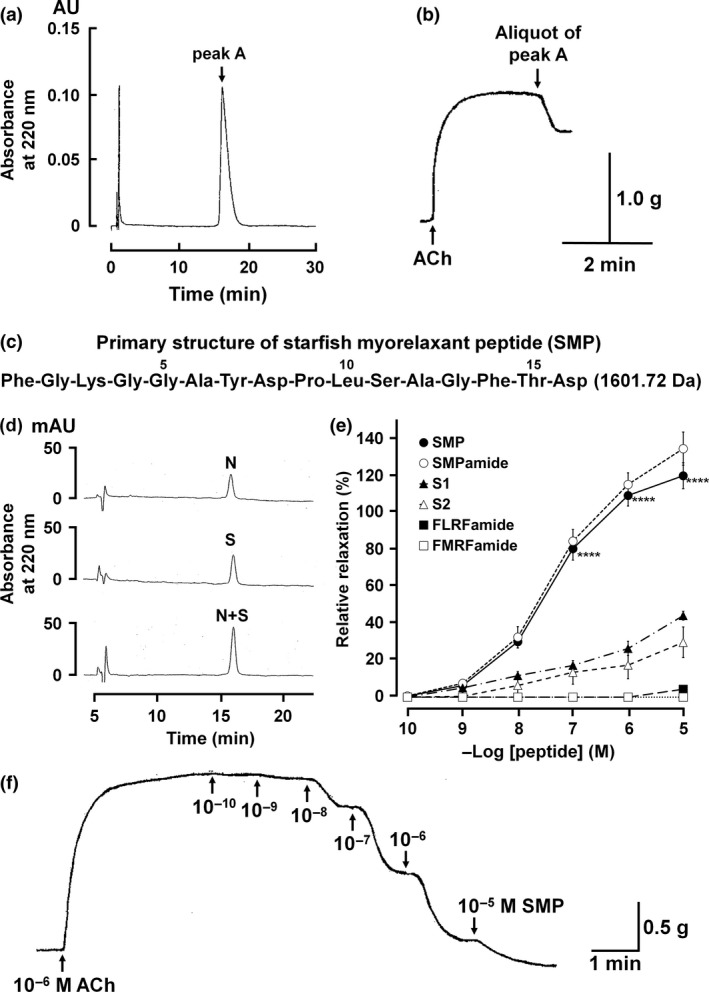
Isolation, structure determination and pharmacology of purified myorelaxant peptide. Peak A was isocratically eluted with 20% acetonitrile/0.1% trifluoroacetic acid on RP‐HPLC (a), and an aliquot of purified peak A caused relaxation of the apical muscle (b). Purified peak A was identified as a peptide comprised of sixteen amino acid residues with a molecular mass of 1601.72 Da, which we have named starfish myorelaxant peptide or SMP (c). Comparison of chromatographic properties of native SMP (N) and synthetic SMP (S) on RP‐HPLC showed that native SMP and synthetic SMP with a free carboxy terminal have identical retention times on RP‐HPLC (d). The concentration‐dependent relaxing activity of SMP on the apical muscle of P. pectinifera. SMP with free carboxyl terminus and amidated carboxy terminus is SMP (●) and SMPamide (○), respectively. The effects of S1 (▲) and S2 (▵) from the starfish A. rubens and the molluscan neuropeptides FLRFamide (■) and FMRFamide (□) are shown to compare their activity with SMP. Each point represents the mean ± standard deviation determined from four separate experiments. ****p < 0.0001 for SMP (●) compared with S1/S2. The percentage relaxing activity was calculated by comparing each relaxation effect to the maximal contraction of the apical muscle by 1 μM ACh (e). Representative recording of the concentration‐dependent relaxing effect of SMP on P. pectinifera apical muscle pre‐contracted with 1 μM ACh (f).
