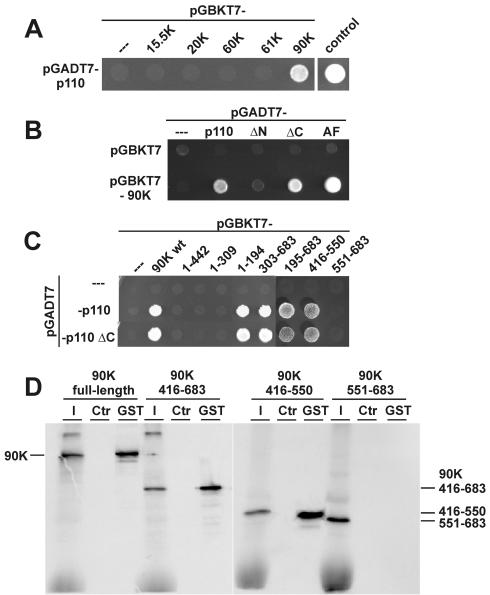
FIG. 5.
p110 interacts through its TPR domain with a C-terminal region (amino acids 416 to 550) of the U4/U6 snRNP-specific 90K protein. (A) Yeast two-hybrid assay of interactions between p110 and U4/U6-specific proteins. Yeast strain AH109 was transformed with plasmid pGBKT7-15.5K, pGBKT7-20K, pGBKT7-60K, pGBKT7-61K, or pGBKT7-90K in combination with plasmid pGADT7-p110. As controls, cotransformations were done with pGADT7-p110 and plasmid pGBKT7 as well as with plasmid pGADT7-T (containing simian virus 40 large T antigen) and plasmid pGBKT7-53 (containing p53). Protein-protein interactions were assayed by selection on minimal synthetic dropout medium lacking leucine, tryptophan, histidine, and adenine for 3 days at 30°C. (B) Yeast two-hybrid assay of interactions between p110 mutant derivatives and the U4/U6-specific 90K protein. Yeast strain AH109 was transformed with plasmid pGADT7-p110, pGADT7-ΔN, pGADT7-ΔC, or pGADT7-AF 387506 (AF) in combination with plasmid pGBKT7-90K. As controls, cotransformations were done with plasmid pGBKT7 or with plasmids pGADT7 and pGBKT7-90K. Selection was done for 3 days at 30°C on minimal synthetic dropout medium lacking leucine, tryptophan, and histidine (SD-LWH medium). (C) Yeast two-hybrid assay of interactions between p110 and U4/U6-specific 90K protein mutant derivatives. Yeast strain AH109 was transformed with plasmid pGADT7, pGADT7-p110, or pGADT7-ΔC. In addition, plasmid pGBKT7-90K or pGBKT7 constructs containing various regions of the 90K protein (amino acids 1 to 442, 1 to 309, 1 to 194, 303 to 683, 195 to 683, 195 to 683, 416 to 550, and 551 to 683) were cotransformed. Interactions were assayed for 3 days at 30°C on SD-LWH medium. wt, wild type. (D) Mutational analysis of the p110-90K protein interaction by GST pull-down assays. 35S-labeled full-length 90K protein and mutant derivatives containing amino acids 416 to 683, 416 to 550, and 551 to 683 were incubated with GST-p110 protein, followed by GST pull-down assays. Coprecipitated proteins were fractionated by SDS-PAGE and visualized by autoradiography. For each assay, 10% of the input (lanes I) and the precipitated material (lanes GST) are shown; control reactions contained only GST protein (lanes Ctr). The mobilities of the 90K full-length and mutant proteins are marked on the left and right.