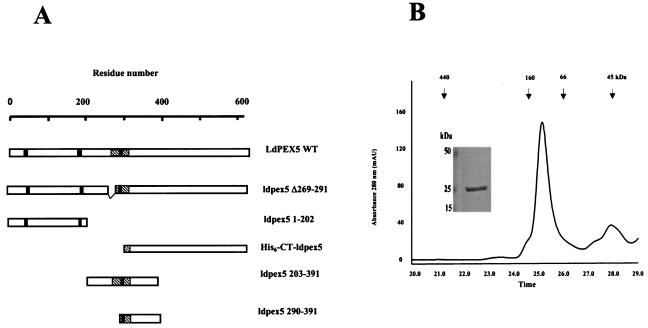
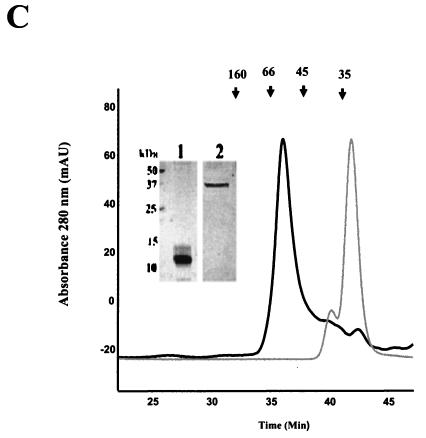
FIG. 1.
Oligomerization domains of LdPEX5. (A) Schematic diagram of the LdPEX5 protein variants used in the present study. The hatched regions in each construct represents the coiled-coil motif, while the black bars denote the three WXXXY/F pentapeptide repeats that are conserved in all PEX5 proteins. (B) The oligomeric state of ldpex5 1-202 was determined on a Bio-Sil SEC 250-5 (7.8 by 600 mm) column as described in Materials and Methods. One-minute fractions were collected, and the proteins in each fraction were analyzed by Coomassie blue-stained SDS-PAGE (numbers at the bottom of the gel correspond to the elution times on the trace). The inset represents a gel analysis of the ∼132-kDa peak eluting at 25 min. SDS-PAGE gel electrophoresis of this peak reveals that it is composed of a protein with a subunit molecular mass of ∼25 kDa. (C) The quaternary structure of the fragments ldpex5 203-391 (thick trace) and ldpex5 290-391 (thin trace) was determined by analysis of 25 μg of each protein on a Superdex 200 HR10/30 column equilibrated with 50 mM Tris (pH 8.0)-100 mM NaCl-2 mM DTT (pH 8.0) at a flow rate of 0.25 ml/min. The inset shows an SDS-PAGE analysis of ldpex5 290-391 (lane 1) and ldepx5 203-391 (lane 2) protein preparations used to perform the gel permeation chromatography experiment. Gel permeation columns were calibrated with protein standard containing a mixture of thyroglobulin (660 kDa), ferritin (440 kDa), bovine IgG (160 kDa), bovine serum albumin (66 kDa), ovalbumin (45 kDa), β-lactoglobulin (35 kDa), or equine myoglobin (17 kDa).