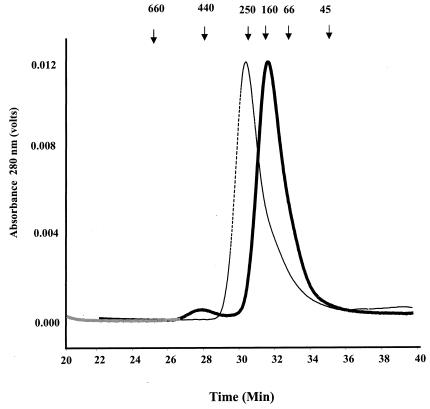
FIG. 3.
Quaternary structure analysis of LdPEX5 proteins. The oligomeric structure of recombinant LdPEX5 proteins and ldpex5 fragments overexpressed in E. coli were assessed by high-pressure liquid gel permeation chromatography. A total of 25 μg of LdPEX5 (thin trace) or ldpex5 Δ269-291 (thick trace) was injected onto a Bio-Sil column equilibrated with the mobile phase 50 mM Tris (pH 8.0)-100 mM NaCl-2 mM DTT at a flow rate of 0.4 ml/min, and protein elution was monitored spectrophotometrically at 280 nm. Gel permeation columns were calibrated with a protein standard containing a mixture of thyroglobulin (660 kDa), ferritin (440 kDa), bovine catalase (250 kDa), bovine IgG (160 kDa), bovine serum albumin (66 kDa), ovalbumin (45 kDa), and equine myoglobin (17 kDa).