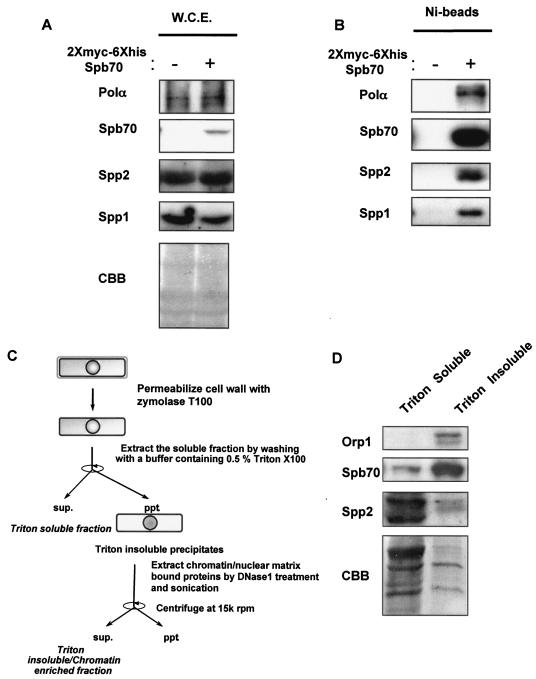
FIG. 1.
Identification of the fission yeast B-subunit of DNA Polα-primase complex. (A) Expression of two-myc-six-His-tagged Spb70 in cells. The two-myc- and six-His-tagged spb70+ and untagged spb70+ strain were grown at 25°C in EMM to mid-log phase. Cells were harvested and whole-cell extracts (W.C.E.) were prepared as described in Materials and Methods. The expression of Polα/p180, Spp2/p58, and Spp1/p49 and absence of two-myc-six-His-tagged Spb70 in whole-cell extracts from cells containing spb70+ with no epitope tag (−) and expression of two-myc-six-His-tagged Spb70 in whole-cell extracts from cells containing the two-myc-six-His-tagged spb70+ (+) are shown by Western blotting with their respective antibodies and anti-myc antibody. CBB, the Coomassie brilliant blue staining of the Western blot membrane. (B) Spb70 physically associates with Polα/p180 and primase Spp1/p49 and Spp2/p58. Ni bead pull-down fractions of whole-cell extracts from the untagged spb70+ strain (−) and from the two-myc-six-His-tagged spb70+ strain (+) were blotted with their respective antibodies. (C) Schematic diagram of cell fractionation. (D) Spb70 localizes in both Triton X-100-soluble and -insoluble fractions. The strain containing two-myc-six-His-spb70+ and HA-orp1+ was grown at 25°C in EMM to mid-log phase. Cells were harvested, and Triton X-100-soluble and -insoluble (chromatin-enriched) fractions were prepared as described in Materials and Methods and outlined in panel C. HA-tagged Orp1 was used as the Triton X-100-insoluble protein control in chromatin-enriched fraction; two-myc-six-His-Spb70 and Spp2/p58 in each fraction was shown by Western blotting with their respective antibodies. As described in Materials and Methods, the intensity of each protein shown in Western blotting does not represent their molar quantities in cell extracts and Ni bead pull-downs due to the difference in reactivity of each antibody. CBB, the Coomassie brilliant blue staining of the immunoblot membrane.