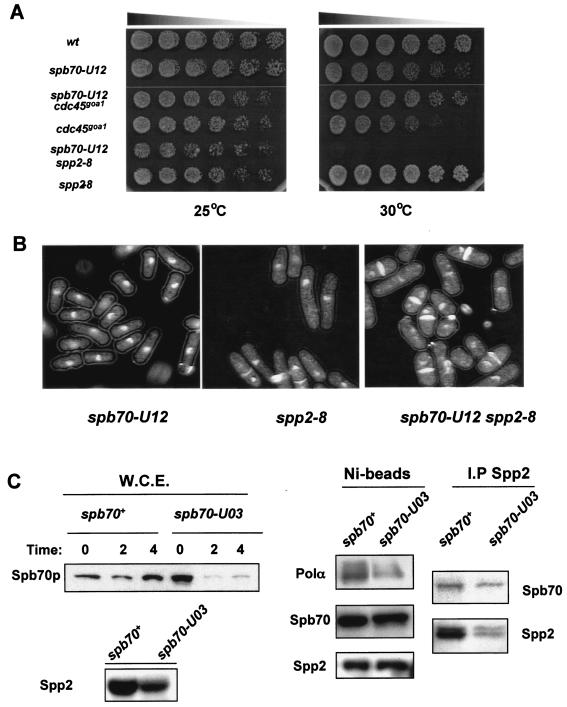
FIG. 3.
Genetic and physical characterizations of spb70 mutants. (A) Genetic interactions. Wild type (wt), single mutants spb70-U12, cdc45goa1, and spp2-8, and double mutants spb70-U12 cdc45goa1 and spb70-U12 spp2-8 were cultured in liquid YES medium at 25°C. Equal numbers of log-phase cells were spotted on YES plates in threefold serial dilution and incubated at 25 and 30°C for 3 days. (B) Terminal phenotypes. Single mutants spb70-U12 and spp2-8 and double mutant spb70-U12 spp2-8 were first grown in liquid YES medium at 25°C then shifted up to 36°C for 4 h. Cells were harvested and stained with DAPI and calcofluor. (C) Stability of Spb70 protein and interaction of Spb70 with other Polα-primase subunits. Wild-type two-myc-six-His-spb70+ and mutant two-myc-six-His-spb70-U03 cells were cultured at 25°C in EMM to mid-log phase and then shifted to 36°C. At the indicated time, cells were harvested and whole-cell extracts (W.C.E.) were prepared. The Spb70 protein levels in whole-cell extracts from spb70+ and spb70-U03 cells were detected by anti-myc antibody (W.C.E. panel). Spp2/p58 protein levels in whole-cell extracts from spb70+ and spb70-U03 cells after the shift to 36°C for 4 h used for the Ni bead pull-down are shown in the bottom panel. Ni bead pull-down fractions from wild-type two-myc-six-His-spb70+ and mutant two-myc-six-His-spb70-U03 whole-cell extracts after shifting the cells to 36°C for 4 h were analyzed by Western blotting with anti-Polα antibody, anti-HA for Spp2/p58, and anti-myc antibody for Spb70 for association of Polα/p180 and Spp2/p58 with Spb70 protein, respectively (Ni-beads panel). HA-tagged Spp2 proteins were immunoprecipitated from whole-cell extracts of the wild type containing two-myc-six-His-spb70+ and spp2+-three-HA and the mutant containing two-myc-six-His-spb70-U03 and spp2+-three-HA with anti-HA antibody. The immunoprecipitates were analyzed by Western blotting with anti-myc for Spb70 and anti-HA antibody for Spp2 (I.P Spp2 panel).