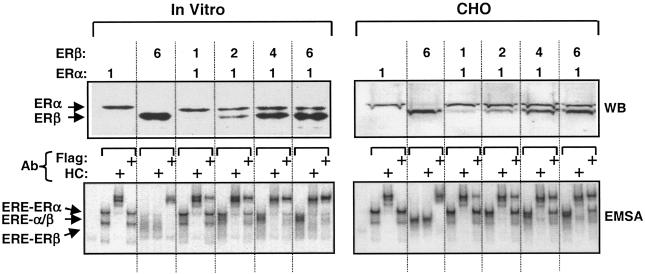
FIG. 2.
Coexpression of ERα and ERβ in vitro and in situ leads to formation of ERα/β heterodimer. For Western blotting of in vitro-synthesized ERs, 0.3 μg of pBS-KS vector bearing the Flag ERα cDNA was coexpressed with 0.3, 0.6, 1.2, or 1.8 μg of vector bearing the Flag-ERβ cDNA that correspond to 1-, 2-, 4-, or 6-fold-greater amounts, respectively, of the expression vector. Equal amounts (5 μl) of reaction mixtures were subjected to SDS-7.5% PAGE, followed by Western blotting with the Flag antibody. Migration of ERα and ERβ are indicated. The dimerization of ERα and ERβ was assessed by EMSA with equal amounts of transcription-translation mixture (5 μl). For Western blotting of in situ-synthesized ERs, 0.5 μg of the mammalian expression vector bearing the Flag-ERα cDNA was cotransfected with 0.5, 1.0, 2.0, or 3.0 μg (corresponding to 1, 2, 4, or 6, respectively) of the expression vector carrying the Flag-ERβ cDNA in CHO cells. Equal amounts of cell extracts (10 μg of total protein) were subjected to SDS-7.5% PAGE, followed by Western blotting with the Flag antibody. For EMSA, the cosynthesis of ERs was accomplished as described above, except that the cDNA for ERα does not contain sequences for the Flag epitope to distinguish ER species by the use of the antibody. Equal amounts of total protein (10 μg) of CHO cell extracts were subjected to EMSA. ERE-ER complexes were analyzed by using the HC or Flag antibody. ERE-bound ERα and ERβ homodimers and the ERα/β heterodimer are indicated. Unbound ERE is not shown.