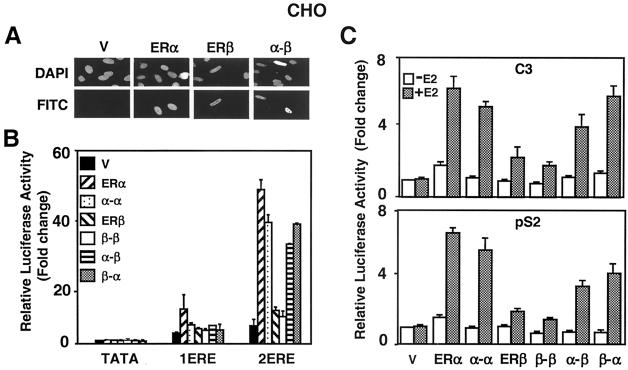
FIG. 7.
(A) Intracellular localization of fusion ERs was examined by immunocytochemistry. The expression vector without (V) or with a cDNA for Flag ERα, ERβ, or fusion α-β was transiently transfected into CHO cells. The proteins were probed with the Flag antibody and visualized by using fluorescein-conjugated secondary antibody (FITC). DAPI (4′,6′-diamidino-2-phenylindole) staining indicates the nucleus. There was no protein detectable in cells transfected with the parent vector. The absence or presence (data not shown) of 10−9 M E2 did not affect the intracellular localization of the ERs. (B) CHO cells were transiently transfected with 75 ng of expression vector bearing a cDNA for an ER, together with 125 ng of reporter plasmid bearing no (TATA), one (1ERE), or two copies of the consensus ERE (2ERE). The TATA box promoter drives the expression of the firefly luciferase cDNA. The transfection efficiency was monitored by determining the coexpression of 2 ng of reporter plasmid bearing the Renilla luciferase cDNA. Cells were treated without (data not shown) or with 10−9 M E2 (E2) for 24 h. The data represent the means ± the standard errors of the mean (SEM) of three independent experiments performed in duplicate. (C) CHO cells transiently transfected as described above by using a reporter plasmid bearing the C3 or pS2 promoter that drive the expression of the firefly luciferase enzyme cDNA. Cells were treated in the absence (−E2) or presence (+E2) of 10−9 M E2 for 24 h. The results from three independent experiments in duplicate are represented as the means ± the SEM.