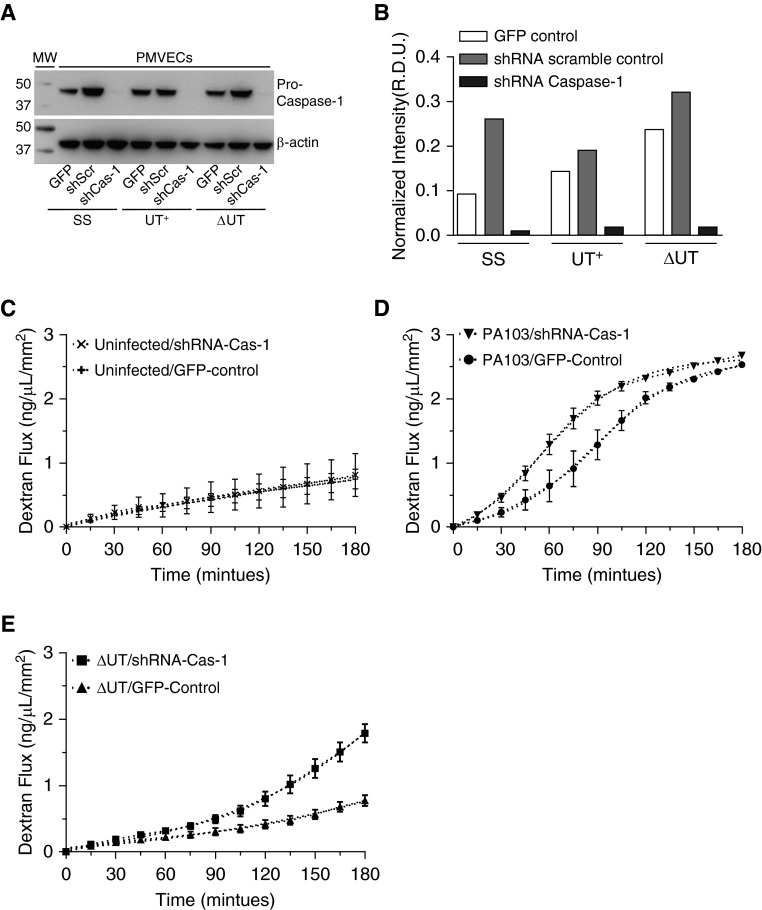
Figure 7.
Genetic knockdown of caspase-1 in cultured rat PMVECs increases the rate of barrier disruption during P. aeruginosa infection. Lentivirus-mediated transduction was used to generate three recombinant PMVEC lines: (1) expressing copGFP reporter alone (GFP); (2) coexpressing copGFP and a control, scrambled shRNA (shScr); and (3) coexpressing copGFP and two shRNAs targeting the caspase-1 mRNA (shCas-1). Recombinant PMVECs grown in culture were untreated (SS) or independently inoculated with either P. aeruginosa PA103(UT+) or the isogenic double-mutant PA103(ΔUT) strain at an MOI of 40 bacteria per host cell. Cells were analyzed after inoculation as described subsequently here. (A) At 3 hours after inoculation, cell lysates were collected and probed with an antibody against caspase-1. Blots were subsequently stripped and reprobed with an antibody against β-actin as a loading control. (B) Representative densitometric analysis of recombinant PMVEC pro–caspase-1 levels was normalized to β-actin. Data are expressed as R.D.U. Representative blots and corresponding densitometry are shown. (C) The recombinant control and shCas-1 PMVECs were grown as monolayers on transwell filters, and transendothelial flux of FITC-dextran was measured over a 180-minute time course. Caspase-1 knockdown did not affect PMVEC basal permeability. (D) Transendothelial FITC-dextran flux of recombinant control and shCas-1 PMVECs was assayed during infection with wild-type PA103(UT+). Compared with control PMVECs, caspase-1 knockdown PMVECs displayed an increased rate of dextran flux when infected with wild-type PA103(UT+), as evidenced by a left shift in the flux curve profile. (E) Transendothelial FITC-dextran flux of recombinant control and shCas-1 PMVECs was assayed during infection with PA103(ΔUT). Compared with control PMVECs, caspase-1 knockdown PMVECs displayed an increased rate of dextran flux when infected with PA103(ΔUT), as evidenced by a left shift in the flux curve profile. (A–E) n = 3 experiments. All data represent mean ± SEM. copGFP, green fluorescent protein 2 from the copepod Pontellina plumata; shRNA, short hairpin RNAs.