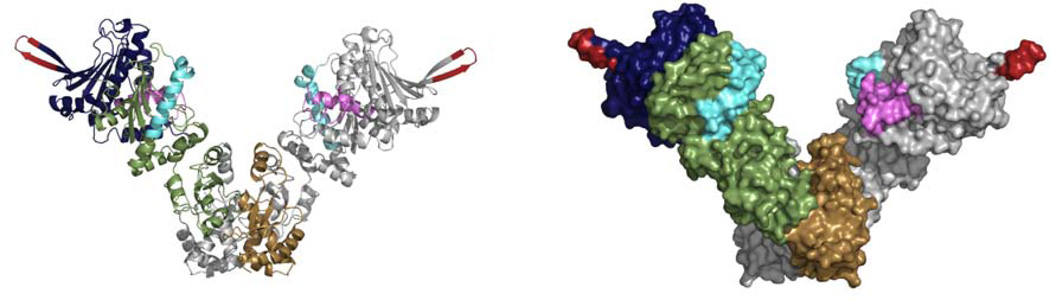
Figure 1.
Hsp90 exists as a homodimer with three domains per monomer. The crystal structure of the bacterial homolog, HtpG (PDB code 2IOQ), is shown as both a cartoon representation and a surface representation. The domains of one monomer are colored blue (NTD), green (MD), and brown (CTD). The other monomer is colored grey. The NTD binds nucleotide and contains a variable charged loop (red) and the conserved lid region (purple) which is adjacent to the nucleotide binding pocket. The middle domain contains the conserved catalytic loop (cyan) which is essential for ATP hydrolysis. The CTD provides the constitutive dimer interface.