Abstract
Purpose
The CYP1B1 gene encodes an enzyme that is a member of the cytochrome P450 superfamily. Mutations in CYP1B1 have been mainly reported in recessive pediatric ocular phenotypes, such as primary congenital glaucoma (PCG) and congenital glaucoma with anterior segment dysgenesis (CG with ASD), with some likely pathogenic variants also identified in families affected with adult-onset primary open angle glaucoma (POAG).
Methods
We examined CYP1B1 in 158 pediatric patients affected with PCG (eight), CG with ASD (22), CG with other developmental ocular disorders (11), juvenile glaucoma with or without additional ocular anomalies (26), and ASD or other developmental ocular conditions without glaucoma (91); in addition, a large cohort of adult patients with POAG (193) and POAG-negative controls (288) was examined.
Results
Recessive pathogenic variants in CYP1B1 were identified in two PCG pedigrees, three cases with CG and ASD, and two families with CG and other ocular defects, such as sclerocornea in one patient and microphthalmia in another individual; neither sclerocornea nor microphthalmia has been previously associated with CYP1B1. Most of the identified causative mutations are new occurrences of previously reported pathogenic alleles with two novel variants identified: a c.1325delC, p.(Pro442Glnfs*15) frameshift allele in a family with PCG and a c.157G>A, p.(Gly53Ser) variant identified in a proband with CG, Peters anomaly, and microphthalmia. Analysis of the family history in the CYP1B1-positive families revealed POAG in confirmed or presumed heterozygous relatives in one family with PCG and two families with ASD/CG; POAG was associated with the c.1064_1076del, p.(Arg355Hisfs*69) allele in two of these pedigrees. Screening of an unrelated POAG cohort identified the same c.1064_1076del heterozygous allele in one individual with sporadic POAG but not in age- and ethnicity-matched POAG-negative individuals. Overall, there was no significant enrichment for mutant alleles in CYP1B1 within the POAG cases compared to the controls.
Conclusions
In summary, these data expand the mutational and phenotypic spectra of CYP1B1 to include two novel alleles and additional developmental ocular phenotypes. The contribution of CYP1B1 to POAG is less clear, but loss-of-function variants in CYP1B1, especially c.1064_1076del, p.(Arg355Hisfs*69), may be associated with an increased risk for POAG.
Introduction
Glaucoma, a degenerative optic neuropathy, represents an important cause of blindness worldwide [1,2]. Primary congenital glaucoma (PCG) is characterized by onset in newborns or diagnosis within the first 2 years of life (also referred to as infantile) and the absence of visible structural ocular anomalies [3]. In many cases, congenital glaucoma is accompanied by anterior segment dysgenesis (ASD), such as iris hypoplasia, iridocorneal adhesions, or Peters anomaly (CG with ASD), thus indicating a defect in the embryonic development of ocular structures. Primary open angle glaucoma (POAG) is the most common form of glaucoma characterized by adult onset and the absence of visible ocular defects. Congenital glaucomas (primary or associated with ASD) are typically inherited as Mendelian traits (autosomal recessive or dominant) while POAG demonstrates a complex inheritance pattern in most pedigrees [4-7].
CYP1B1 (OMIM# 601771) is a member of the cytochrome P450 superfamily and encodes a monooxygenase involved in the metabolism of a broad range of endogenous and exogenous substrates. Mutations in CYP1B1 have been shown to cause various glaucoma phenotypes [7]. The role of CYP1B1 in PCG is well established with homozygous or compound heterozygous missense or truncating variants reported in more than 600 patients [7-10]. Homozygous or compound heterozygous mutations in CYP1B1 have also been reported in patients with ASD, including Peters anomaly, Axenfeld-Rieger anomaly, ectropion uveae with partial aniridia, and even complete aniridia [11-18]; the majority of these patients also had congenital glaucoma. Significant allelic heterogeneity exists with more than 150 distinct mutations already reported [9,10]. Phenotypic heterogeneity is also seen with juvenile open angle glaucoma (JOAG)/POAG occasionally reported in individuals with homozygous pathogenic variants in CYP1B1 [19,20]. Heterozygous variants in CYP1B1, primarily missense alleles, have been reported in sporadic and familial POAG cases [21-30].
CYP1B1 mutant proteins demonstrate decreased enzymatic activity or decreased protein stability [24,31-34]. Studies in mice revealed abnormalities in the trabecular meshwork of Cyp1b1-deficient animals, including irregular collagen distribution, increased oxidative stress, and decreased levels of periostin (Postn), a secreted extracellular matrix protein expressed in collagen-rich tissues, including the trabecular meshwork. Studies of trabecular meshwork cells from human patients with glaucoma confirmed increased oxidative stress and decreased levels of Postn, suggesting a possible mechanism for the glaucoma phenotype in patients with mutations in CYP1B1 [35]. Recent functional characterization of missense mutations in CYP1B1 identified that variants associated with PCG typically affected the metabolism of retinol, thus disrupting the level of retinoic acid that is known to be critical for ocular development, while variants associated with POAG affected the metabolism of 17β-estradiol, which may contribute to POAG through MYOC (OMIM# 601652) overexpression or increased levels of reactive oxygen species and apoptosis [36,37]. Of note, some variants affected both processes and were associated with both types of glaucoma while other variants specifically affected only one or the other. To further define the contribution of CYP1B1 to human glaucoma phenotypes, as well as to investigate its possible role in other ocular conditions, we screened CYP1B1 in patients with various developmental ocular phenotypes, as well as a cohort affected with POAG.
Methods
Human subjects
This human study was approved by the Institutional Review Board of the Children’s Hospital of Wisconsin and adhered to the tenets of the Declaration of Helsinki and ARVO guidelines. Written informed consent was obtained for every subject; in addition, informed consent was obtained for all individual participants whose photographs are included. We screened DNA from 158 probands with developmental ocular conditions for variants in CYP1B1, including eight with PCG, 22 with ASD and congenital glaucoma, 11 with other developmental ocular disorders (including microphthalmia, anophthalmia, coloboma, sclerocornea, and cataract) and congenital glaucoma, three with isolated juvenile glaucoma, 19 with ASD/juvenile glaucoma, four with other developmental ocular disorders and juvenile glaucoma, 50 with ASD without glaucoma, and 41 with other developmental ocular phenotypes without glaucoma. For this study, PCG was used for congenital glaucoma without visible structural ocular anomalies while ASD included diagnoses such as Axenfeld-Rieger anomaly, Peters anomaly, iris hypoplasia, pupil anomalies, and generalized anterior segment dysgenesis that did not fit a specific diagnosis. Patients were from a variety of racial and ethnic backgrounds. Many patients were additionally screened for mutations in PITX2 (OMIM 601542), FOXC1 (OMIM 601090), FOXE3 (OMIM 601094), BG3LCT (OMIM 610308), and other genes with no pathogenic alleles identified [38-40]. The full CYP1B1 coding sequence was obtained for all probands; all available family members were screened for the specific mutations identified in the proband.
In addition, 193 adults with POAG diagnosed by their ophthalmologists from the Marshfield Clinic Personalized Medicine Research Project (PMRP) were screened. The PMRP project was reviewed and approved by the Institutional Review Board of Marshfield Clinic, and all participants provided written informed consent. This population has been described previously [41]. One case was diagnosed in childhood (12.2 years); the age of diagnosis for the remainder ranged from 29.8 to 86.8 years with an average age of 61.2 years. The population was primarily Caucasian (184); 115 cases were female, and 78 were male. In addition, 288 controls from the PMRP were screened. Controls from the PMRP all had normal ophthalmic exams within the past 3 years. Age ranged from 29.4 to 84.8 years with an average age of 55.3 years. The control population was primarily Caucasian (280); 169 cases were female, and 119 were male. Variant frequencies in the general population were obtained from the Exome Aggregation Consortium [42].
CYP1B1 mutation screen
Blood or buccal samples were obtained from each participant and genomic DNA was extracted using Qiagen reagents/kits (Quiagen, Valencia, CA) and standard protocols. Whenever possible, samples were extracted within 24-48 h of draw and refrigerated prior to extraction. The entire coding region and the exon–intron junctions of CYP1B1 (reference sequence NM_000104.3) were screened with direct DNA sequencing of PCR products in the cases and controls. The CYP1B1 coding region was amplified using the following primers: set 1 forward, 5'- TCT CCA GAG AGT CAG CTC CG-3', and set 1 reverse, 5'- GGG TCG TCG TGG CTG TAG-3', and set 2 forward, 5'- ATG GCT TTC GGC CAC TAC T-3', and set 2 reverse, 5'- GAT CTT GGT TTT GAG GGG TG-3', and set 3 forward 5’- TCC CAG AAA TAT TAA TTT AGT CAC TG-3’ and set 3 reverse 5’- TAT GGA GCA CAC CTC ACC TG-3’. PCR products were sequenced in both directions with standard conditions and 20% betaine using Big Dye Terminator v3.1 (Applied Biosystems, Foster City, CA) with 3730XL DNA Analyzer (Applied Biosystems, Foster City, CA). Chromatograms were examined manually and using Mutation Surveyor software (SoftGenetics, State College, PA). All initially identified changes were confirmed with additional independent PCR and sequencing experiments. In silico analysis of the effect of missense mutations was performed using SIFT, PolyPhen2, MutationTaster, MutationAssessor, and FATHMM prediction algorithms accessed through the SNP & Variation Suite software package (SVS; Golden Helix, Bozeman, MT) using data from dbNSFP 2.9 [43].
Results
Mutations in CYP1B1 in pediatric phenotypes
Homozygous or compound heterozygous pathogenic variants in CYP1B1 were identified in seven families affected with the following phenotypes: PCG (Patients 1, 2), congenital glaucoma with ASD (Patients 3–5), sclerocornea and congenital glaucoma (Patient 6), and microphthalmia, Peters anomaly, and congenital glaucoma (Patient 7; Table 1, Figure 1, Figure 2). Eight pathogenic variants were identified; two of these alleles were novel (c.1325delC p.(Pro442Glnfs*15) and c.157G>A p.(Gly53Ser); Table 1), and the remaining six have each been previously reported as causative mutations in congenital glaucoma [9,10]. All of the variants are extremely rare (<0.09% allele frequency) or novel with the exception of the c.1103G>A p.(Arg368His) variant, which was present in 0.3% of Caucasian and 2.9% of South Asian alleles in ExAC with ten homozygotes present in this general population database. This variant has been seen more than 90 times in patients with PCG [9,10], and incomplete penetrance has been reported in multiple families with homozygous or compound heterozygous missense mutations in CYP1B1 [12,44,45].
Table 1. Pediatric phenotypes and CYP1B1 genotypes.
| Patient# | Race/ ethnicity | CYP1B1 mutationa | Functional predictionb | Allele frequencyc | # homozygote controlsd | Eye | Family history |
|---|---|---|---|---|---|---|---|
| 1 |
Caucasian (U.S./Spain) |
c.1064_1076del p.(Arg355Hisfs*69) (mat) |
Premature truncation |
NP |
0 |
Bilateral PCG |
Brother with PCG; several relatives with adult-onset glaucoma; distant cousin with PCG |
| c.1159G>A p.(Glu387Lys) (pat) |
Damaging by 5/5 (S, PP, MT, MA, F) |
36/64286 |
0 |
||||
| 2 |
S. Asian (Pakistan) |
homozygous c.1325delC p.(Pro442Glnfs*15) (mat + pat) |
Premature truncation |
1/16512 |
0 |
Bilateral PCG |
Extensive family history of PCG |
| 3 |
Hispanic (U.S.) |
c.535delG p.(Ala179Argfs*18) (pat) |
Premature truncation |
0/1080 |
0 |
Bilateral CG, right form fruste Axenfeld anomaly (synechia and pupil eccentricity) |
Maternal grandmother with adult-onset glaucoma |
| c.1064_1076del p.(Arg355Hisfs*69) (mat) |
Premature truncation |
NP |
0 |
||||
| 4 |
Caucasian (Turkey) |
homozygous c.1103G>A p.(Arg368His)
(mat + pat) |
Damaging by 4/5 (S, PP, MT, MA) |
184/63292 |
9 SA
1 Eu |
Bilateral CG, iris hypoplasia, posterior embryotoxon |
Consanguinity; maternal grandmother with vision loss later in life |
| 5 |
Caucasian (U.S.) |
c.182G>A p.(Gly61Glu) (pat) |
Damaging by 5/5 (S, PP, MT, MA, F) |
22/26024 |
0 |
Bilateral infantile glaucoma (<1 year of age) and iris hypoplasia |
Father with mild iris dysplasia; paternal great grandfather with adult-onset glaucoma |
| c.1064_1076del p.(Arg355Hisfs*69) |
Premature truncation |
NP |
0 |
||||
| 6 |
Caucasian (Iran) |
homozygous
c.182G>A, p.(Gly61Glu)
(mat + pat) |
Damaging by 5/5 (S, PP, MT, MA, F) |
22/26024 |
0 |
Bilateral CG and sclerocornea |
Consanguinity; no history of ocular disorders |
| 7 | Caucasian (Lebanon) |
c.157G>A p.(Gly53Ser) (mat) |
Damaging by 5/5 (S, PP, MT, MA, F) |
NP |
0 |
Bilateral CG and Peters anomaly, right microphthalmia | Consanguinity; no history of ocular disorders |
| c.1405C>T, p.(Arg469Trp) (pat) | Damaging by 4/5 (S, PP, MT, MA) | 5/66734 | 0 |
PCG=Primary congenital glaucoma; CG=Congenital Glaucoma; ASD=Anterior segment dysgenesis; NP=Not Present; (mat): mutation present in the mother, (pat): mutation present in the father; novel pathogenic alleles and phenotypic features are shown in bold font a Nucleotide numbering is relative to reference sequence NM_000104.3 where +1 is the A of the ATG initiation codon b Five prediction algorithms (SIFT (S), PolyPhen2 (PP), MutationTaster (MT), MutationAssessor (MA), FATHMM (F)) from dbNSFP 2.9 were accessed through SNP & Variation Suite (Golden Helix, Bozeman, MT) c Allele frequency for most closely related ethnic populations provided (European, S. Asian or Latino); in ExAC (Exome Aggregation Consortium; EXAC) d Number of homozygotes present in ExAC is noted Eu: European, SA: South Asian,
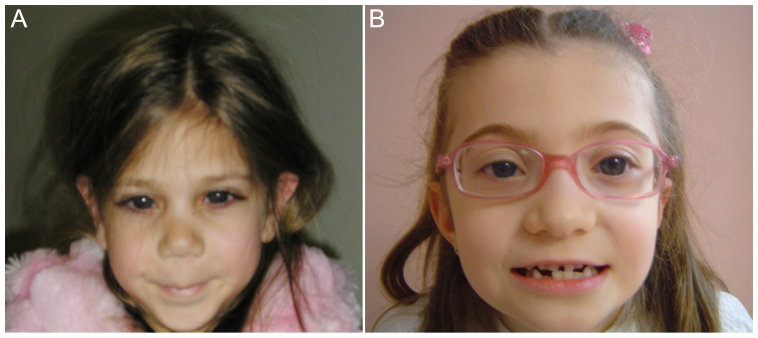
Figure 1.
Photographs of patients. A: Photograph of Patient 1 with bilateral primary congenital glaucoma taken at 5 years of age. B: Photograph of Patient 4 with congenital glaucoma and anterior segment dysgenesis at 6 years of age.
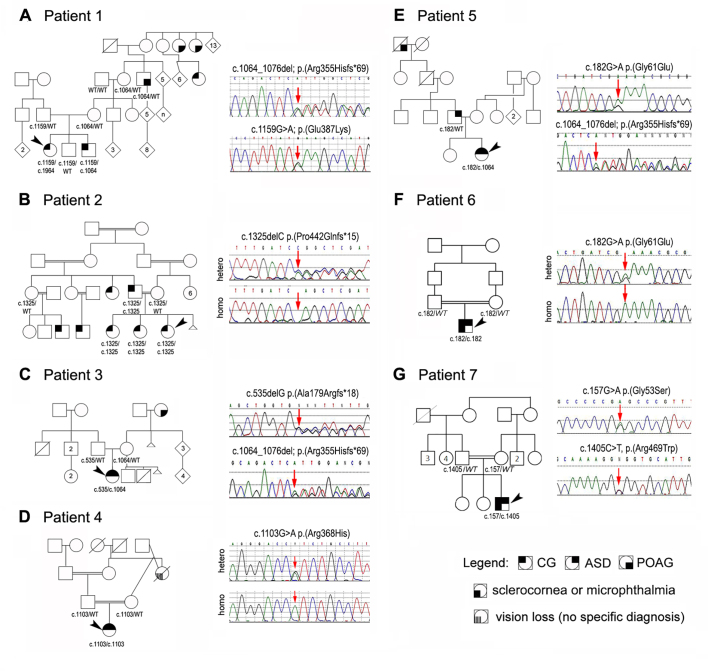
Figure 2.
Pedigrees and CYP1B1 mutation information. Patients 1–7 are indicated with black arrowheads in each pedigree (A-G). DNA sequencing chromatograms of CYP1B1 pathogenic alleles are shown, and variant positions are indicated with red arrows.
The novel frameshift mutation, c.1325delC, p.(Pro442Glnfs*15), identified in Patient 2, is a truncating variant located within the meander region of the protein; this allele cosegregates with the disease phenotype in the affected family and is seen in only 1 in 1,6512 South Asian alleles [42]. The novel missense mutation, c.157G>A, p.(Gly53Ser), seen in Patient 7, affects a highly conserved glycine within the hinge region of CYP1B1 and is predicted to affect protein function by all five functional effect prediction programs; this allele is present in trans with a second pathogenic mutation and is not seen in the general population [42].
Two patients presented with features not previously described for CYP1B1. Patient 6 presented with bilateral congenital glaucoma with sclerocornea and a homozygous c.182G>A, p.(Gly61Glu) mutation previously reported in patients with PCG [10]. Patient 7, with the novel c.157G>A, p.(Gly53Ser) and previously reported c.1405C>T, p.(Arg469Trp) mutations, is affected with microphthalmia and opacified cornea in the right eye, anterior staphyloma of the left eye, bilateral type II Peters anomaly, and bilateral congenital glaucoma. He underwent numerous surgeries, including bilateral cornea transplant, lensectomy, and retinal detachment repair, but ultimately lost vision in both eyes.
A family history of PCG was present in two cases (Patients 1 and 2); the identified variants were shown to cosegregate with the phenotype in both families (Figure 2A,B). In all but one family, both parental samples were available for testing. All unaffected parents were found to be heterozygous for the variant seen in their child, and one affected parent (Family 2) was homozygous for the same mutation seen in his child. Three of the seven probands with congenital glaucoma in this study (Patients 1, 3, and 5) have a relative in their grandparents’ or great-grandparents’ generation reported to have POAG (age of diagnosis ranged from 44 to 60 years), and an additional proband (Patient 4) has a grandparent with vision loss later in life of unknown cause. In two families, the c.1064_1076del mutation appears to be involved with the mutation confirmed in an affected relative in one family and present in the daughter of the affected relative in the other (Figure 2A,C).
No homozygous or compound heterozygous pathogenic mutations were seen in patients with juvenile glaucoma with or without ASD/other developmental ocular conditions (26), ASD without glaucoma (50), or other developmental ocular conditions without glaucoma (41). Three heterozygous variants of uncertain significance (c.241T>A, p.(Tyr81Asn), c.1328C>G, p.(Arg443Gly), and c.1462G>C, p.(Ala488Pro)) were identified in patients with variable anterior segment defects or microphthalmia (Appendix 1). Finally, the previously reported c.685G>A, p.(Glu229Lys) heterozygous variant was identified in five pediatric probands without glaucoma (1.6% allele frequency) matching the overall allele frequency of 1.4% in the general population (ExAC), adding further evidence that this is a benign polymorphism. Previous functional analysis of this variant also suggested that this variant did not affect CYP1B1 activity [37].
Mutations in CYP1B1 in POAG
Screening of 193 POAG cases identified an individual who was heterozygous for the c.1064_1076del, p.(Arg355Hisfs*69) allele that was found to be associated with POAG in two pediatric pedigrees reported here. In addition, three other rare heterozygous variants were identified: c.241T>A, p.(Tyr81Asn) and c.1328C>G, p.(Arg443Gly), which were both previously reported in POAG [21,46], and a novel c.35C>T (p.Pro12Leu) variant (Table 2). Two of these variants, p.(Arg443Gly) and (p.Pro12Leu), were predicted to be benign by all five prediction algorithms, and one, p.(Tyr81Asn), was computed to be damaging by four of the five programs (Table 2). None of the cases carried a second mutation in CYP1B1. All four patients are Caucasian, the age of diagnosis ranged from 41 to 63 years of age, and all four patients were treated with medications to lower intraocular pressure (IOP) only (no surgical intervention). A family history of adult-onset glaucoma was noted in Patient 11, but no details were available.
Table 2. Summary of CYP1B1 variants in POAG cases and age-matched controls.
| Case | CYP1B1 mutationa | Functional predictionb | Allele frequencyc | #homozygote controlsd | POAG | Agee | Cup/disc ratio | Family history |
|---|---|---|---|---|---|---|---|---|
| Patient 8 |
c.1064_1076del p.(Arg355Hisfs*69) |
Premature truncation |
NP |
0 |
Yes |
47 years |
0.6/0.7 |
None |
| Patient 9 |
c.241T>A p.(Tyr81Asn) |
Damaging by 4/5 (S, PP, MT, MA) |
159/22812 |
1 Eu, 2 SA, 1 Fn |
Yes |
41 years |
0.4/0.6 |
None |
| Patient 10 |
c.1328C>G p.(Arg443Gly) |
Benign by 5/5 |
86/66736 |
14 Af |
Yes |
63 years |
0.85/
0.875 |
Unknown |
| Patient 11 |
c.35C>T (p.Pro12Leu) |
Benign by 5/5 |
4/62318 |
1 Af |
Yes |
51 years |
0.4/0.75 |
Positive, details unknown |
| Control 1 |
c.1103G>A (p.Arg368His) |
Damaging by 4/5 (S, PP, MT, MA) |
184/63292 |
9 SA, 1 Eu |
No |
57 years |
- |
- |
| Control 2 |
c.1103G>A (p.Arg368His) |
Damaging by 4/5 (S, PP, MT, MA) |
184/63292 |
9 SA, 1 Eu |
No |
60 years |
- |
- |
| Control 3 | c.1405C>T (p.Arg469Trp) | Damaging by 4/5 (S, PP, MT, MA) | 5/66734 | 0 | No | 71 years | - | - |
POAG: Primary open angle glaucoma; NP=Not Present; hom- homozygotes; a Nucleotide numbering is relative to reference sequence NM_000104.3 where +1 is the A of the ATG initiation codon b Five prediction algorithms (SIFT (S), PolyPhen2 (PP), MutationTaster (MT), MutationAssessor (MA), FATHMM (F)) from dbNSFP 2.9 were accessed through SNP & Variation Suite (Golden Helix, Bozeman, MT) c Allele frequency for Caucasian populations in ExAC (Exome Aggregation Consortium; EXAC) d Number of homozygotes present in ExAC is noted; Af: African, Eu: European, Fn: Finnish, SA: South Asian e Age at diagnosis (for Patients 8-11) or at last normal eye exam (for Controls 1-3)
Among the age- and ethnicity-matched controls without glaucoma, heterozygous previously reported pathogenic variants in CYP1B1 associated with recessive PCG were identified in three control individuals, including the c.1103G>A; p.(Arg368His) variant in two and the c.1405C>T; p.(Arg469Trp) variant in one.
The previously reported rare c.685G>A, p.(Glu229Lys) variant and six common previously reported polymorphisms, g.3793T>C (c.-1–12C>T); c.142C>G, p.(Arg48Gly); c.355G>T, p.(Ala119Ser); c.1294G>C, p.(Leu432Val); c.1347T>C, p.(Asp449Asp); c.1358A>G, p.(Asn453Ser), were observed in the cases and controls at similar frequencies (Appendix 2).
Discussion
Homozygous or compound heterozygous mutations in CYP1B1 were identified in a high proportion of probands with PCG (2/8; 25%) and a significant portion of those with congenital glaucoma and ASD (3/22; 14%) or other developmental phenotypes (2/11; 18%). The expansion of the CYP1B1 phenotypic spectrum to the more complex developmental ocular phenotypes of sclerocornea and microphthalmia seems to be consistent with the gene’s predicted broad function. Cyp1b1 demonstrates wide expression during development in animal models [47,48]. CYP1B1 was shown to be involved in retinoic acid (RA) synthesis [48,49], and functional analysis of variants associated with PCG showed abnormal metabolism of retinol by mutant proteins [37]. Mutations of other genes in the retinoic acid pathway are known to cause isolated and syndromic anophthalmia or microphthalmia [50]. Therefore, expanded ocular phenotypes associated with mutations in CYP1B1 are not surprising. At the same time, as these additional anomalies occurred in consanguineous pedigrees, it is possible that other inherited recessive variants contributed to these phenotypes.
Most of the identified mutations in the pediatric cohort have been previously associated with congenital glaucoma and/or ASD. The two novel mutations detected in Patient 2 (c.1325delC, p.(Pro442Glnfs*15)) and Patient 7 (c.157G>A, p.(Gly53Ser)) show strong evidence for pathogenicity. In terms of recurrent mutations, the c.182G>A, p.(Gly61Glu) allele exhibits a possible association with ASD and other ocular phenotypes as this variant was present in two probands with ASD or sclerocornea reported here, as well as in 11 previously published cases with Peters anomaly (five), ectropion uveae with partial aniridia (five), and/or acquired peripheral iris degeneration (one) [12,15,51].
The contribution of CYP1B1 to POAG is less clear. Although variants in CYP1B1 were not found to make a significant contribution to POAG in the unselected cohort screened here, heterozygous loss-of-function variants appear likely to increase the risk of POAG. First, in this report, multiple CYP1B1 pedigrees with congenital phenotypes demonstrated adult-onset glaucoma in heterozygous (or presumed heterozygous) relatives of probands with congenital glaucoma, a novel and interesting finding. A heterozygous loss-of-function allele, c.1064_1076del, p.(Arg355Hisfs*69), was identified in the unselected POAG population and in two families with POAG in relatives of a proband affected with congenital glaucoma. The same heterozygous frameshift mutation was also reported in two French unrelated POAG probands [21], a different heterozygous frameshift mutation (c.1311–1315del5) was reported in one Indian patient with JOAG [23], and two other heterozygous nonsense mutations (c.171G>A, p.Trp59* and c.1084C>T, p.Gln362*) were reported in three other families with JOAG/POAG; family history was noted for one family, in which a sibling with pseudoexfoliative glaucoma carried the same truncating mutation [24,52,53]. None of these truncating mutations were observed in age-matched non-glaucoma controls.
Although two recent meta-analyses found no association between CYP1B1 polymorphisms and POAG [54,55], these studies analyzed common variants with allele frequencies between 15% and 62% and thousands of homozygotes present in the general population, not rare loss-of-function alleles as identified in this study. Previous functional analyses of CYP1B1 mutant proteins suggested that variants that affect the metabolism of 17β-estradiol may contribute to POAG through MYOC overexpression when 17β-estradiol metabolism is decreased or through increased levels of reactive oxygen species and apoptosis when metabolism is increased [36,37]. For many of these variants, the decreased enzyme activity was shown to correlate with decreased protein stability leading to decreased levels of protein; consistent with this, the frameshift mutation would be expected to result in decreased levels of functional CYP1B1 protein. Given the rarity of loss-of-function variants (as demonstrated by the rarity of PCG in the general population), it is perhaps not surprising that loss-of-function variants in CYP1B1 would explain only a small proportion of the much more common POAG. Although many factors, both genetic and environmental, are involved in POAG pathogenesis, a heterozygous loss-of-function variant in CYP1B1, particularly one that affects the metabolism of 17β-estradiol seems likely to confer an increased risk of glaucoma in adulthood. Further study and characterization of missense alleles is needed to determine which missense variants affect CYP1B1 function (and thus may confer an increased risk of glaucoma) and which are benign variants.
In summary, CYP1B1 represents a major gene for primary congenital glaucoma and a significant contributor to congenital glaucoma associated with ASD. In addition, the phenotype associated with mutations in CYP1B1 has been expanded to include sclerocornea and microphthalmia, but the gene does not appear to be involved in developmental ocular phenotypes that do not include congenital glaucoma. Although CYP1B1 does not appear to be a major contributor to POAG in the general population, the presence of the same heterozygous frameshift variant, c.1064_1076del, p.(Arg355Hisfs*69), in an unselected POAG proband and two CG pedigrees with a family history of POAG, as well as the identification of POAG in a relative of a third CG proband, suggests that some CYP1B1 pathogenic variants increase the risk for POAG. Until more is known about which alleles are associated with increased risk, heterozygous carrier relatives of patients with pediatric CYP1B1 phenotypes should be monitored for adult-onset glaucoma.
Acknowledgments
We are grateful to the patients and families for participating in this study. This project was supported by awards from National Institutes of Health/ National Eye Institute [grant number R01EY015518 to EVS] and funds provided by the Children’s Research Institute Foundation at Children’s Hospital of Wisconsin grant along with P30EY001931 Core Grant from the National Institutes of Health, 1UL1RR031973 from the Clinical and Translational Science Award (CTSA) program, and the American Health Assistance Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix 1.
Summary of heterozygous CYP1B1 variants of uncertain significance and patient features. To access the data, click or select the words “Appendix 1.”
Appendix 2.
Summary of CYP1B1 polymorphisms in PMRP POAG cases and non-POAG controls. To access the data, click or select the words “Appendix 2.”
References
- 1.Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15640920&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 2.Rudnicka AR, Mt-Isa S, Owen CG, Cook DG, Ashby D. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Invest Ophthalmol Vis Sci. 2006;47:4254–61. doi: 10.1167/iovs.06-0299. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17003413&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 3.Ho CL, Walton DS. Primary congenital glaucoma: 2004 update. J Pediatr Ophthalmol Strabismus. 2004;41:271–88. doi: 10.3928/01913913-20040901-11. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15478740&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 4.Libby RT, Gould DB, Anderson MG, John SW. Complex genetics of glaucoma susceptibility. Annu Rev Genomics Hum Genet. 2005;6:15–44. doi: 10.1146/annurev.genom.6.080604.162209. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16124852&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 5.Sowden JC. Molecular and developmental mechanisms of anterior segment dysgenesis. Eye (Lond) 2007;21:1310–8. doi: 10.1038/sj.eye.6702852. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17914434&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 6.Allingham RR, Liu Y, Rhee DJ. The genetics of primary open-angle glaucoma: a review. Exp Eye Res. 2009;88:837–44. doi: 10.1016/j.exer.2008.11.003. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19061886&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasiliou V, Gonzalez FJ. Role of CYP1B1 in glaucoma. Annu Rev Pharmacol Toxicol. 2008;48:333–58. doi: 10.1146/annurev.pharmtox.48.061807.154729. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17914928&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 8.Stoilov I, Akarsu AN, Sarfarazi M. Identification of three different truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (Buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Hum Mol Genet. 1997;6:641–7. doi: 10.1093/hmg/6.4.641. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9097971&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 9.de Melo MB, Mandal AK, Tavares IM, Ali MH, Kabra M, de Vasconcellos JP, Senthil S, Sallum JM, Kaur I, Betinjane AJ, Moura CR, Paula JS, Costa KA, Sarfarazi M, Paolera MD, Finzi S, Ferraz VE, Costa VP, Belfort R, Jr, Chakrabarti S. Genotype-Phenotype Correlations in CYP1B1-Associated Primary Congenital Glaucoma Patients Representing Two Large Cohorts from India and Brazil. PLoS ONE. 2015;10:e0127147. doi: 10.1371/journal.pone.0127147. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25978063&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li N, Zhou Y, Du L, Wei M, Chen X. Overview of Cytochrome P450 1B1 gene mutations in patients with primary congenital glaucoma. Exp Eye Res. 2011;93:572–9. doi: 10.1016/j.exer.2011.07.009. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21854771&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 11.Vincent A, Billingsley G, Priston M, Williams-Lyn D, Sutherland J, Glaser T, Oliver E, Walter MA, Heathcote G, Levin A, Heon E. Phenotypic heterogeneity of CYP1B1: mutations in a patient with Peters' anomaly. J Med Genet. 2001;38:324–6. doi: 10.1136/jmg.38.5.324. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11403040&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edward D, Al Rajhi A, Lewis RA, Curry S, Wang Z, Bejjani B. Molecular basis of Peters anomaly in Saudi Arabia. Ophthalmic Genet. 2004;25:257–70. doi: 10.1080/13816810490902648. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15621878&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 13.Chavarria-Soley G, Michels-Rautenstrauss K, Caliebe A, Kautza M, Mardin C, Rautenstrauss B. Novel CYP1B1 and known PAX6 mutations in anterior segment dysgenesis (ASD). J Glaucoma. 2006;15:499–504. doi: 10.1097/01.ijg.0000243467.28590.6a. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17106362&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 14.Tanwar M, Dada T, Dada R. Axenfeld-Rieger Syndrome Associated with Congenital Glaucoma and Cytochrome P4501B1 Gene Mutations. Case Rep Med. 2010;2010:212656. doi: 10.1155/2010/212656. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20827438&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan AO, Aldahmesh MA, Alkuraya FS. Genetic and genomic analysis of classic aniridia in Saudi Arabia. Mol Vis. 2011;17:708–14. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21423868&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 16.Khan AO, Aldahmesh MA, Mohamed JY, Alkuraya FS. Corneal enlargement without optic disk cupping in children with recessive CYP1B1 mutations. J AAPOS. 2013;17:643–5. doi: 10.1016/j.jaapos.2013.08.004. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24210336&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 17.Prokudin I, Simons C, Grigg JR, Storen R, Kumar V, Phua ZY, Smith J, Flaherty M, Davila S, Jamieson RV. Exome sequencing in developmental eye disease leads to identification of causal variants in GJA8, CRYGC, PAX6 and CYP1B1. Eur J Hum Genet. 2014 doi: 10.1038/ejhg.2013.268. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24281366&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelberman D, Islam L, Jacques TS, Russell-Eggitt I, Bitner-Glindzicz M, Khaw PT, Nischal KK, Sowden JC. CYP1B1-related anterior segment developmental anomalies novel mutations for infantile glaucoma and von Hippel's ulcer revisited. Ophthalmology. 2011;118:1865–73. doi: 10.1016/j.ophtha.2011.01.044. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21600657&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 19.Safari I, Suri F, Haji-Seyed-Javadi R, Yazdani S, Elahi E. The p.Gly61Glu Mutation in CYP1B1 Affects the Extracellular Matrix in Glaucoma Patients. Ophthalmic Res. 2016 doi: 10.1159/000443508. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26982174&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 20.Bashir R, Tahir H, Yousaf K, Naz S, Naz S. Homozygous p.G61E mutation in a consanguineous Pakistani family with co-existence of juvenile-onset open angle glaucoma and primary congenital glaucoma. Gene. 2015;570:295–8. doi: 10.1016/j.gene.2015.07.014. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26164761&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 21.Melki R, Colomb E, Lefort N, Brezin AP, Garchon HJ. CYP1B1 mutations in French patients with early-onset primary open-angle glaucoma. J Med Genet. 2004;41:647–51. doi: 10.1136/jmg.2004.020024. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15342693&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Garrido MP, Sanchez-Sanchez F, Lopez-Martinez F, Aroca-Aguilar JD, Blanco-Marchite C, Coca-Prados M, Escribano J. Heterozygous CYP1B1 gene mutations in Spanish patients with primary open-angle glaucoma. Mol Vis. 2006;12:748–55. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16862072&dopt=Abstract [PubMed] [Google Scholar]
- 23.Chakrabarti S, Devi KR, Komatireddy S, Kaur K, Parikh RS, Mandal AK, Chandrasekhar G, Thomas R. Glaucoma-associated CYP1B1 mutations share similar haplotype backgrounds in POAG and PACG phenotypes. Invest Ophthalmol Vis Sci. 2007;48:5439–44. doi: 10.1167/iovs.07-0629. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18055790&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 24.Pasutto F, Chavarria-Soley G, Mardin CY, Michels-Rautenstrauss K, Ingelman-Sundberg M, Fernandez-Martinez L, Weber BH, Rautenstrauss B, Reis A. Heterozygous loss-of-function variants in CYP1B1 predispose to primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2010;51:249–54. doi: 10.1167/iovs.09-3880. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19643970&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 25.Vincent AL, Billingsley G, Buys Y, Levin AV, Priston M, Trope G, Williams-Lyn D, Heon E. Digenic inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. Am J Hum Genet. 2002;70:448–60. doi: 10.1086/338709. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11774072&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acharya M, Mookherjee S, Bhattacharjee A, Bandyopadhyay AK, Daulat Thakur SK, Bhaduri G, Sen A, Ray K. Primary role of CYP1B1 in Indian juvenile-onset POAG patients. Mol Vis. 2006;12:399–404. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16688110&dopt=Abstract [PubMed] [Google Scholar]
- 27.Bayat B, Yazdani S, Alavi A, Chiani M, Chitsazian F, Tusi BK, Suri F, Narooie-Nejhad M, Sanati MH, Elahi E. Contributions of MYOC and CYP1B1 mutations to JOAG. Mol Vis. 2008;14:508–17. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18385784&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 28.Suri F, Kalhor R, Zargar SJ, Nilforooshan N, Yazdani S, Nezari H, Paylakhi SH, Narooie-Nejhad M, Bayat B, Sedaghati T, Ahmadian A, Elahi E. Screening of common CYP1B1 mutations in Iranian POAG patients using a microarray-based PrASE protocol. Mol Vis. 2008;14:2349–56. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19096718&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 29.Micheal S, Ayub H, Zafar SN, Bakker B, Ali M, Akhtar F, Islam F, Khan MI, Qamar R, den Hollander AI. Identification of novel CYP1B1 gene mutations in patients with primary congenital and primary open-angle glaucoma. Clin Experiment Ophthalmol. 2015;43:31–9. doi: 10.1111/ceo.12369. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25091052&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 30.Gong B, Qu C, Li X, Shi Y, Lin Y, Zhou Y, Shuai P, Yang Y, Liu X, Zhang D, Yang Z. Mutation spectrum of CYP1B1 in Chinese patients with primary open-angle glaucoma. Br J Ophthalmol. 2015;99:425–30. doi: 10.1136/bjophthalmol-2014-306054. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25527694&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 31.Chavarria-Soley G, Sticht H, Aklillu E, Ingelman-Sundberg M, Pasutto F, Reis A, Rautenstrauss B. Mutations in CYP1B1 cause primary congenital glaucoma by reduction of either activity or abundance of the enzyme. Hum Mutat. 2008;29:1147–53. doi: 10.1002/humu.20786. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18470941&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 32.Choudhary D, Jansson I, Sarfarazi M, Schenkman JB. Characterization of the biochemical and structural phenotypes of four CYP1B1 mutations observed in individuals with primary congenital glaucoma. Pharmacogenet Genomics. 2008;18:665–76. doi: 10.1097/FPC.0b013e3282ff5a36. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18622259&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 33.Campos-Mollo E, Lopez-Garrido MP, Blanco-Marchite C, Garcia-Feijoo J, Peralta J, Belmonte-Martinez J, Ayuso C, Escribano J. CYP1B1 mutations in Spanish patients with primary congenital glaucoma: phenotypic and functional variability. Mol Vis. 2009;15:417–31. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19234632&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Garrido MP, Blanco-Marchite C, Sanchez-Sanchez F, Lopez-Sanchez E, Chaques-Alepuz V, Campos-Mollo E, Salinas-Sanchez A, Escribano J. Functional analysis of CYP1B1 mutations and association of heterozygous hypomorphic alleles with primary open-angle glaucoma. Clin Genet. 2010;77:70–8. doi: 10.1111/j.1399-0004.2009.01284.x. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19793111&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, Wang S, Sorenson CM, Teixeira L, Dubielzig RR, Peters DM, Conway SJ, Jefcoate CR, Sheibani N. Cyp1b1 mediates periostin regulation of trabecular meshwork development by suppression of oxidative stress. Mol Cell Biol. 2013;33:4225–40. doi: 10.1128/MCB.00856-13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23979599&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mookherjee S, Acharya M, Banerjee D, Bhattacharjee A, Ray K. Molecular basis for involvement of CYP1B1 in MYOC upregulation and its potential implication in glaucoma pathogenesis. PLoS ONE. 2012;7:e45077. doi: 10.1371/journal.pone.0045077. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23028769&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banerjee A, Chakraborty S, Chakraborty A, Chakrabarti S, Ray K. Functional and Structural Analyses of CYP1B1 Variants Linked to Congenital and Adult-Onset Glaucoma to Investigate the Molecular Basis of These Diseases. PLoS ONE. 2016;11:e0156252. doi: 10.1371/journal.pone.0156252. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27243976&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reis LM, Tyler RC, Volkmann Kloss BA, Schilter KF, Levin AV, Lowry RB, Zwijnenburg PJ, Stroh E, Broeckel U, Murray JC, Semina EV. PITX2 and FOXC1 spectrum of mutations in ocular syndromes. Eur J Hum Genet. 2012;20:1224–33. doi: 10.1038/ejhg.2012.80. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22569110&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reis LM, Tyler RC, Schneider A, Bardakjian T, Stoler JM, Melancon SB, Semina EV. FOXE3 plays a significant role in autosomal recessive microphthalmia. Am J Med Genet A. 2010;152A:582–90. doi: 10.1002/ajmg.a.33257. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20140963&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weh E, Reis LM, Tyler RC, Bick D, Rhead WJ, Wallace S, McGregor TL, Dills SK, Chao MC, Murray JC, Semina EV. Novel B3GALTL mutations in classic Peters plus syndrome and lack of mutations in a large cohort of patients with similar phenotypes. Clin Genet. 2014;86:142–8. doi: 10.1111/cge.12241. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23889335&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCarty CA, Mukesh BN, Kitchner TE, Hubbard WC, Wilke RA, Burmester JK, Patchett RB. Intraocular pressure response to medication in a clinical setting: the Marshfield Clinic Personalized Medicine Research Project. J Glaucoma. 2008;17:372–7. doi: 10.1097/IJG.0b013e31815c5f3f. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18703947&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 42.ExAC. Exome Aggregation Consortium. Cambridge, MA. Available at: http://exac.broadinstitute.org. Accessed June, 2016.
- 43.Liu X, Jian X, Boerwinkle E. dbNSFP v2.0: a database of human non-synonymous SNVs and their functional predictions and annotations. Hum Mutat. 2013;34:E2393–402. doi: 10.1002/humu.22376. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23843252&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen L, Huang L, Zeng A, He J. CYP1B1 gene mutations with incomplete penetrance in a Chinese pedigree with primary congenital glaucoma: a case report and review of literatures. Int.J.Clin.Exp.Med. 2015;8:14538–41. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26550445&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 45.Suri F, Yazdani S, Narooie-Nejhad M, Zargar SJ, Paylakhi SH, Zeinali S, Pakravan M, Elahi E. Variable expressivity and high penetrance of CYP1B1 mutations associated with primary congenital glaucoma. Ophthalmology. 2009;116:2101–9. doi: 10.1016/j.ophtha.2009.04.045. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19744731&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 46.Milla E, Mane B, Duch S, Hernan I, Borras E, Planas E, Dias Mde S, Carballo M, Gamundi MJ. Spanish Multicenter Glaucoma Group-Estudio Multicentrico Espanol de Investigacion Genetica del Glaucoma, EMEIGG. Survey of familial glaucoma shows a high incidence of cytochrome P450, family 1, subfamily B, polypeptide 1 (CYP1B1) mutations in non-consanguineous congenital forms in a Spanish population. Mol Vis. 2013;19:1707–22. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23922489&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 47.Stoilov I, Rezaie T, Jansson I, Schenkman JB, Sarfarazi M. Expression of cytochrome P4501b1 (Cyp1b1) during early murine development. Mol Vis. 2004;10:629–36. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15359218&dopt=Abstract [PubMed] [Google Scholar]
- 48.Chambers D, Wilson L, Maden M, Lumsden A. RALDH-independent generation of retinoic acid during vertebrate embryogenesis by CYP1B1. Development. 2007;134:1369–83. doi: 10.1242/dev.02815. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17329364&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 49.Chen H, Howald WN, Juchau MR. Biosynthesis of all-trans-retinoic acid from all-trans-retinol: catalysis of all-trans-retinol oxidation by human P-450 cytochromes. Drug Metab Dispos. 2000;28:315–22. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10681376&dopt=Abstract [PubMed] [Google Scholar]
- 50.Reis LM, Semina EV. Conserved genetic pathways associated with microphthalmia, anophthalmia, and coloboma. Birth Defects Res C Embryo Today. 2015;105:96–113. doi: 10.1002/bdrc.21097. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26046913&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan AO, Aldahmesh MA, Mohamed JY, Alkuraya FS. Congenital glaucoma with acquired peripheral circumferential iris degeneration. J AAPOS. 2013;17:105–7. doi: 10.1016/j.jaapos.2012.09.011. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23363883&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 52.Micheal S, Ayub H, Zafar SN, Bakker B, Ali M, Akhtar F, Islam F, Khan MI, Qamar R, den Hollander AI. Identification of novel CYP1B1 gene mutations in patients with primary congenital and primary open-angle glaucoma. Clin Experiment Ophthalmol. 2015;43:31–9. doi: 10.1111/ceo.12369. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25091052&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 53.Patel HY, Richards AJ, De Karolyi B, Best SJ, Danesh-Meyer HV, Vincent AL. Screening glaucoma genes in adult glaucoma suggests a multiallelic contribution of CYP1B1 to open-angle glaucoma phenotypes. Clin Experiment Ophthalmol. 2012;40:e208–17. doi: 10.1111/j.1442-9071.2011.02714.x. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22004014&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 54.Dong S, Yang J, Yu W, Kota P, Xia X, Xu H. No association of genetic polymorphisms in CYP1B1 with primary open-angle glaucoma: a meta- and gene-based analysis. Mol Vis. 2012;18:786–96. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22509109&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Z, Li M, Li L, Sun H, Lin XY. Association of single nucleotide polymorphisms in the CYP1B1 gene with the risk of primary open-angle glaucoma: a meta-analysis. Genet Mol Res. 2015;14:17262–72. doi: 10.4238/2015.December.16.26. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26681220&dopt=Abstract [DOI] [PubMed] [Google Scholar]