Abstract
Background
Malaria contributes to elevated morbidity and mortality in populations displaced by conflict in tropical zones. In an attempt to reduce malaria transmission in an internally displaced persons (IDP) camp in eastern Democratic Republic of Congo (DRC), we tested a strategy of active case detection of household contacts of malaria cases.
Methods
Prospective community-based survey.
Results
From a convenience sample of 100 febrile patients under 5 years of age from the IDP camp presenting to a nearby clinic for management of a fever episode, 19 cases of uncomplicated malaria and 81 controls with non-malarial febrile illness (NFMI) were diagnosed. We engaged community health workers in the IDP camp to screen their household contacts for malaria using rapid diagnostic tests. We detected 29 cases of malaria through this active case-finding procedure. Household contacts of children with uncomplicated malaria were no more likely to have positive Plasmodium falciparum antigenemia than controls with NFMI (OR 0.89, 95% CI 0.33 to 2.4, p = 1.0), suggesting that malaria cases did not cluster at the household level. However, household contacts reporting mild symptoms at the time of community survey (headache, myalgia) had a higher odds of malaria than asymptomatic individuals (OR 14 (95% CI 4.2–48), p ≤ 0.001 and 18 (95% CI 5.9–54), p ≤ 0.001, respectively).
Conclusion
Screening household contacts of malaria cases was not an efficient case-finding strategy in a Congolese IDP camp. Symptom-based screening may be a simpler and cost-effective method to identify individuals at increased risk of malaria for targeted screening and treatment in an IDP camp.
Keywords: Malaria, Internally displaced persons, Screening, Cost-effectiveness, Febrile illness
Introduction
Malaria claims 1.24 million lives annually, mostly children in sub-Saharan Africa.1 Malaria is particularly problematic in complex humanitarian emergencies (CHEs) in the tropics, including conflict areas sheltering internally displaced persons (IDPs). Malaria morbidity and mortality increases during and after periods of violent conflict, in areas such as the Democratic Republic of the Congo (DRC). A survey in eastern DRC showed that during a period when violent deaths increased 5.5-fold, malaria-specific mortality increased 3.5-fold.2
Detection and treatment of malaria cases is a pillar of malaria control.3 Relying on passive case detection of patients who present to health facilities may miss a reservoir of infected individuals who do not seek health care because they are asymptomatic or minimally symptomatic.4 Strategies to reach asymptomatically infected individuals include mass drug administration (MDA) and mass screening and treatment (MSAT).5 Active case detection through MSAT is resource-intensive; more selective application of screening may increase efficiencies, as in other targeted infectious disease control measures.6−8 Malaria cases also tend to be spatially and temporally clustered,9 suggesting a potential role for selective screening and treatment of household contacts. In one study from Zambia, there was a higher prevalence of asymptomatic infections found in households of patients with clinical malaria diagnosed in a health facility.10 This suggests that household contact tracing may be an efficient mechanism to identify malaria infections, in order to administer treatment and reduce the parasite reservoir.
In the setting of a Congolese IDP camp with high malaria burden but few resources for MDA or MSAT, we hypothesized that household contacts (i.e. temporary shelter co-habitants) of patients with malaria would be at higher risk of malaria than controls (household contacts of children with non-malarial febrile illness [NMFI]). We tested this hypothesis in a prospective study and compared screening of household contacts to strategies of MDA, MSAT, and symptom-based screening.
Methods
This was a prospective cohort study in a Congolese IDP camp. The primary analysis tested the hypothesis that household contacts of children with malaria have a higher prevalence of parasite antigenemia than household contacts of children with non-malarial febrile illness.
As a secondary analysis, we examined other risk factors for prevalent parasite antigenemia among the cohort of household contacts and explored how we could exploit these factors to design a cost-effective active case finding strategy.
Participants and setting
We conducted the study in the IDP camp of Bulengo, near the village of Mugunga, in Karisimbi district, North Kivu province, Eastern DRC. The eastern regions of the DRC have faced years of civil and international conflict resulting in large-scale population displacement. A recent survey of mortality in Eastern DRC found a crude mortality rate above the emergency threshold; furthermore, the main cause of death of children under five was due to fever/malaria.11 The camp occupies a land area of approximately 1.5 km2 near Lake Kivu, 17 km south of Goma, the capital city of North Kivu province. This camp housed an estimated 45,000–50,000 temporary residents at the time of the study. The settlement has existed for more than 4 years, with several waves of displaced persons from numerous villages in the territories of Masisi, Rutshuru, and Walikale (all in North Kivu province). Displacement from their ancestral lands was associated with violent conflict involving a complex array of militarized factions divided along political and ethnic lines (e.g. Hutu, Tutsi, Nande, Nyanga, and Hunde). Camp inhabitants live in tent-like temporary structures made from tarpaulin and local materials (thatch).
Clinic-based recruitment of index cases and NFMI controls
We enrolled index cases (children with uncomplicated malaria) and controls (children with NMFI) from the only health clinic in the area, which provides free services to IDP camp residents. A convenience sample of 100 children from the IDP camp presenting for management of febrile illness was tested for malaria using a histidine-rich protein 2 (HRP2)-based RDT (Paracheck-Pf, Orchid Biomedical, India). All participants who tested positive for malaria were treated with artemisinin-based combination therapy (ACT) in accordance with World Health Organization recommendations [23]. The accompanying parent or guardian underwent a structured interview pertaining to the child’s condition leading up to the clinic visit as well as general questions regarding their education and family assets.
Community-based follow-up of household contacts
Following training and verification of knowledge and skills in the safe and reliable use of RDTs, community health workers were distributed RDTs and malaria treatment (ACT). They conducted follow-up visits at the family shelter in the IDP camp to administer a questionnaire and to screen the household contacts using RDT. Malaria positive cases were treated with ACT.
Ethics, consent, and permissions
In the clinic, the accompanying parent or guardian provided verbal consent for their child to participate in the study. At the time of contact tracing, individual household members also provided verbal consent to participate in the study. We obtained ethical permission for the study from the Comitée d’Éthique du Nord Kivu (Université Catholique du Graben, Butembo, DRC), the University of Alberta Health Research Ethics Board, and the regional Médecin Chef de Zone (Medical Officer of the Zone).
Statistical analysis
We used chi-squared test for binomial variables and Mann–Whitney U-test for continuous variables to compare household-level and individual-level characteristics between contacts of malaria cases and NFMI controls. Statistical analyses were conducted using STATA 12.1 (StataCorp LP, College Station, TX).
To create a statistical model of the hierarchical (nested) data of individuals within households, we used R (R Core Team, version 3.1.2, 2014) and lme4 to perform a generalized linear mixed-effects analysis (hierarchical logistical model) of the malaria risk among household contacts. As fixed effects, we entered age, sex, malaria status of the index case, and symptoms of the contact at the time of survey (headache or myalgia) into the model. We modeled intercepts for each household as random effects. Visual inspection of residual plots did not reveal any obvious deviations from homoscedasticity or normality. The p-value for the primary hypothesis (malaria risk among household contacts of index children with malaria versus index children with NMFI) was obtained by likelihood ratio test of the full model (including malaria status of the index case) against the model without malaria status of the index case.
Results and discussion
Tracing of household contacts
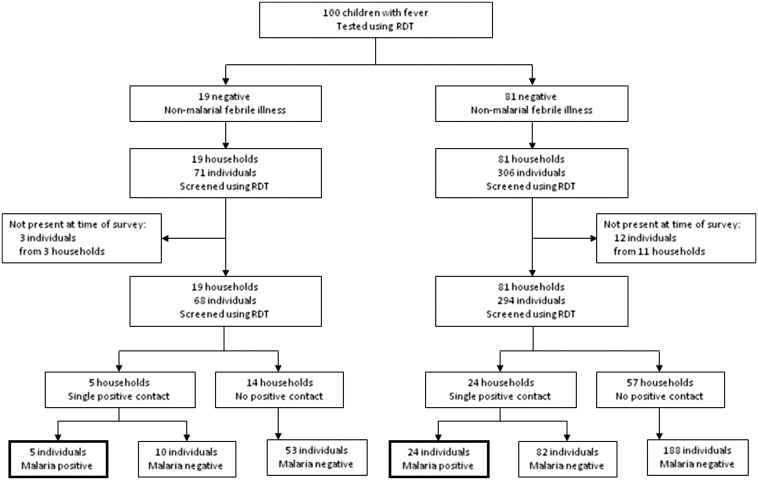
We prospectively tested 100 children presenting to the Bulengo IDP camp clinic with febrile illness for malaria between 27 November 2013 and 9 January 2014 (Figure 1). We found 19 cases of uncomplicated malaria and 81 cases of NMFI. We visited the households of these 19 children and tested 68 household contacts between 28 November 2013 and 10 January 2014. Three individuals (4.2%) were reported missing from the house at the time of the survey. Five individuals from five different households tested positive for malaria. As a control group, we visited the households of 81 children with NMFI and tested 294 household contacts, of whom 24 individuals from 24 different households tested positive for malaria. Twelve individuals (3.9%) were reported missing from the household at the time of the survey. Thus, a positive household contact was found for 5/19 (26%) index cases with malaria, compared to 24/81 (29%) of controls with NMFI [odds ratio (OR) 0.85 (95%CI 0.27–2.6), p = 1.0]. The proportion of household contacts of index cases with malaria who tested positive for malaria was 5/68 (7.4%), compared to 24/294 (8.2%) household contacts of controls with NMFI [OR 0.89 (95%CI 0.33–2.4), p = 1.0]. Household characteristics were no different between malaria cases and NMFI controls, including bed net ownership (Table 1). We next tested whether malaria risk differed between household contacts of children with malaria and household contacts of children with NMFI using a hierarchical logistic multivariate model. Adjusting for age, sex, symptoms (headache or myalgia), and accounting for the hierarchical (household-clustered) structure of the data, malaria risk did not differ between contacts of children with malaria and NMFI controls [OR 0.23 (95%CI 0.050 to 2.3), p = 0.66]. Therefore, it appeared that malaria transmission did not cluster at the household level, and screening household contacts with malaria did not have higher diagnostic yield compared to controls. This finding contrasts with previous studies from Zambia and Swaziland, where malaria prevalence was higher among household contacts of malaria cases.9 Important differences between our study population and the Zambian and Swaziland cohorts that may explain a lack of household clustering include holoendemic vs unstable transmission dynamics, higher density of housing in the IDP camp and social disruption in the CHE.
Figure 1.
Flowchart of the prospective study. One hundred febrile children presenting for care were tested for malaria using point-of-care rapid diagnostic tests. Nineteen tested positive (malaria cases) and 81 tested negative (non-malarial febrile illness (NFMI) controls). Community health workers conducted door-to-door follow-up of the households of all the participants, testing all household contacts, and found 5 new positive malaria cases among household contacts of malaria cases and 24 cases among contacts of NFMI controls (OR 0.89, 95% CI 0.33 to 2.4, p = 1.0).
Table 1.
Characteristics of households of index cases screened with RDTs (active case finding)
| Households of index case with uncomplicated malaria (m = 19) | Households of index case with non-malarial febrile illness (m = 81) | p-value | |
|---|---|---|---|
| Total number of household contacts screened | 68 | 294 | |
| Household Size, median (IQR) | |||
| Total | 5 (4–5.5) | 5 (3–6) | 0.78 |
| Children under 5 | 1 (1–2) | 1 (1–2) | 0.58 |
| Length of stay in IDP camp [months], median (IQR) | 13 (9–15) | 13 (12–26) | 0.58 |
| Household Asset ownership, m (%) | |||
| Bicycle | 7 (37) | 16 (20) | 0.12 |
| Car | 0 | 0 | 1.00 |
| Radio | 4 (21) | 23 (28) | 0.58 |
| Telephone | 5 (26) | 29 (36) | 0.41 |
| Television | 0 | 0 | 1.00 |
| Fridge | 0 | 0 | 1.00 |
| Electricity | 0 | 0 | 1.00 |
| Cement construction | 1 (5) | 0 | 0.19 |
| Chicken | 5 (26) | 10 (12) | 0.13 |
| Cow | 1 (5) | 1 (1) | 0.35 |
| Goat | 4 (21) | 7 (9) | 0.21 |
| Household bed net ownership, m (%) | 4 (21) | 13 (16) | 0.73 |
| Education of female head of household, m (%) | |||
| No education | 11 (58) | 54 (67) | |
| Primary | 6 (32) | 24 (30) | |
| Secondary | 2 (11) | 3 (4) | 0.40 |
| University | 0 (0) | 0 (0) | |
| Malaria infection in the household, m/M (%) | 5/19 (26) | 24/81 (29) | 1.0 |
| Household contacts with malaria, n/N (%) | 5/68 (7.4) | 24/294 (8.2) | 1.0 |
Alternative methods for identification of malaria cases in the IDP camp
Given similar malaria infection rates in household contacts of both malaria cases and NMFI controls, we pooled household contacts in a secondary analysis of other possible risk factors of malaria infection (Table 2). We found that self-reported symptoms (headache and myalgia) were associated with RDT positivity [OR 14 (95% CI 4.2–47.6), p ≤ 0.001 and 18 (95% CI 5.9–53.6), p ≤ 0.001, respectively]. These associations remained highly significant in a hierarchical logistic multivariable model adjusting for the nested structure of the data and for co-variates (age, sex, malaria status of index case). Of note, the diagnosis of these patients occurred outside the clinic setting, during household visits in the camp, and they had not presented for medical care. This suggested an alternative pre-screening strategy using self-reported malaria symptoms. A cost comparison of MDA, MSAT, and alternative strategies of screening household contacts of malaria cases and symptom-based pre-screening is shown in Table 3. The most cost-effective strategy is screening symptomatic patients within the camp, whereas MDA and MSAT are the most comprehensive strategies (fewest missed cases). Screening household contacts is neither cost-effective nor comprehensive since malaria did not cluster at the household level in this cohort.
Table 2.
Characteristics of household contacts screened for malaria with RDTs
| Malaria test positive, n = 29a | Malaria test negative, n = 333a | p-value | |
|---|---|---|---|
| Age (yr), median (IQR) | 14 (8–22) | 19 (8–27) | 0.39 |
| Female sex, n (%) | 16 (55) | 183 (55) | 0.98 |
| Symptoms, n (%) | |||
| Fever | 1 (3) | 1 (0) | 0.15 |
| Headache | 6 (21) | 6 (2) | <0.001 |
| Myalgia | 8 (28) | 8 (2) | <0.001 |
| Malaria episode within the last month, n (%) | 0 (0) | 6 (2) | 1.00 |
| Fever within the past month | 2 (7) | 9 (3) | 0.28 |
Fifteen household contacts of index cases were not present during follow-up visit.
Table 3.
Comparison of strategies for detection and antimalarial drug administration for malaria cases within IDP camp that did not present to clinic
| No. of RDTs used | No. of appropriate treatments | No. of unnecessary treatments | No. of missed cases | Direct cost of RDT & medication | Cost per case detected & treated | |
|---|---|---|---|---|---|---|
| Mass Drug Administration (MDA) | 0 | 29 | 333 | 0 | $1267 | $44 |
| Screen and treat if RDT+ | ||||||
| Screen All (MSAT) | 362 | 29 | 0 | 0 | $667 | $23 |
| Screen if symptomatic | 30 | 15 | 0 | 14 | $98 | $7 |
| Screen household contacts of child with malaria | 68 | 5 | 0 | 24 | $120 | $24 |
This study has several limitations. It was conducted at a single, densely populated IDP camp where malaria transmission is intense and year-round; therefore, results (e.g. lack of household clustering) should not be extrapolated to lower transmission settings, sparsely populated areas, or areas with marked seasonal transmission. Social determinants including population displacement, extreme poverty, and low uptake of bednets (17% household ownership in this study) are somewhat unique to the IDP camp setting and results may not be generalizable to stable, more wealthy populations. On the other hand, this study provides valuable and rare data for this distinct, vulnerable and difficult to study population. Our sampling strategy (household contacts of children with fever) was appropriate to test our hypothesis that malaria cases would cluster within households (primary analysis). Our secondary analysis would ideally involve a random subset of the IDP camp. Our simplified cost analysis does not exhaustively compare all direct and indirect costs of various active case-finding strategies; instead, it was intended to provide a comparison of the methods in terms of comprehensiveness and cost.
Conclusions
The elevated malaria morbidity and mortality in IDP camps demands a pro-active, resource-efficient response. In addition to well-known malaria control methods such as MDA and MSAT, symptom-based pre-screening (headache, myalgia) may rapidly identify IDP camp residents in whom RDT testing will have a high diagnostic yield and who may represent an unreached reservoir for onward transmission. This cost-effective active find-and-treat strategy deserves further study to investigate whether malaria incidence in an IDP camp can be reduced using this method.
List of abbrevations
- ACD
active case detection
- ACT
Artemisinin combination therapy
- CHE
complex humanitarian emergencies
- DRC
Democratic Republic of the Congo
- HRP
Histidine-rich protein
- IDP
internally displaced persons
- MDA
mass drug administration
- MSAT
mass screening and treatment
- NMFI
non-malarial febrile illness
- RDT
rapid diagnostic test
Competing interests
The authors declare that they have no competing interests.
Funding
This work was supported by Association For Health Innovation in Africa
Acknowledgments
This study was funded through the Association For Health Innovation in Africa (AFHIA), a registered charitable organization in the DRC.
References
- 1.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: global burden of disease study. Lancet. 1997;349(9061):1269–1276. 10.1016/S0140-6736(96)07493-4 [DOI] [PubMed] [Google Scholar]
- 2.Conroy AL, Phiri H, Hawkes M, Glover S, Mallewa M, Seydel KB, et al. . Endothelium-based biomarkers are associated with cerebral malaria in malawian children: a retrospective case-control study. PLoS One. 2010;5(12):e15291 10.1371/journal.pone.0015291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiff C. Integrated approach to malaria control. Clin Microbiol Rev. 2002;15(2):278–293. 10.1128/CMR.15.2.278-293.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alves FP, Gil LH, Marrelli MT, Ribolla PE, Camargo EP, Da Silva LH. Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J Med Entomol. 2005;42(5):777–779. 10.1603/0022-2585(2005)042[0777:ACOPSA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 5.Macauley C. Aggressive active case detection: a malaria control strategy based on the Brazilian model. Soc Sci Med. 2005;60(3):563–573. 10.1016/j.socscimed.2004.05.025 [DOI] [PubMed] [Google Scholar]
- 6.Kault DA. Modelling AIDS reduction strategies. Int J Epidemiol. 1995;24(1):188–197. 10.1093/ije/24.1.188 [DOI] [PubMed] [Google Scholar]
- 7.Langenskiold E, Herrmann FR, Luong BL, Rochat T, Janssens JP. Contact tracing for tuberculosis and treatment for latent infection in a low incidence country. Swiss Med Wkly. 2008;138(5–6):78–84. [DOI] [PubMed] [Google Scholar]
- 8.Hagan JE, Smith W, Pillai SK, Yeoman K, Gupta S, Neatherlin J, et al. . Implementation of Ebola case-finding using a village chieftaincy taskforce in a remote outbreak – Liberia, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(7):183–185. [PMC free article] [PubMed] [Google Scholar]
- 9.Sturrock HJ, Hsiang MS, Cohen JM, Smith DL, Greenhouse B, Bousema T, et al. . Targeting asymptomatic malaria infections: active surveillance in control and elimination. PLoS Med. 2013;10(6):e1001467 10.1371/journal.pmed.1001467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stresman GH, Kamanga A, Moono P, Hamapumbu H, Mharakurwa S, Kobayashi T, et al. . A method of active case detection to target reservoirs of asymptomatic malaria and gametocyte carriers in a rural area in Southern Province, Zambia. Malar J. 2010;8 p.; Article ID: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrión Martín AI, Bil K, Salumu P, Baabo D, Singh J, Kik C, et al. . Mortality rates above emergency threshold in population affected by conflict in North Kivu, Democratic Republic of Congo, July 2012–April 2013. PLoS Negl Trop Dis. 2014;8(9):e3181 10.1371/journal.pntd.0003181 [DOI] [PMC free article] [PubMed] [Google Scholar]