Abstract
Toll-like receptors (TLRs) play a critical role in the innate immune response against pathogens. Each TLR recognizes specific pathogen-associated molecular patterns, after which they activate the adaptor protein MyD88 or TRIF-assembled signaling complex to produce immune mediators, including inflammatory cytokines and type I IFNs. Although the activation of TLR is important for host defense, its uncontrolled activation can damage the host. During the past decade, numerous studies have demonstrated that GSK3β is a key regulator of inflammatory cytokine production in MyD88-mediated TLR signaling via TLR2 and TLR4. Recently, GSK3β has also been implicated in the TRIF-dependent signaling pathway via TLR3. In this review, we describe current advances on the regulatory role of GSK3β in immune responses associated with various TLRs. A better understanding of the role of GSK3β in TLR signaling might lead to more effective anti-inflammatory interventions. [BMB Reports 2016; 49(6): 305-310]
Keywords: Glycogen synthase kinase 3β (GSK3β), Inflammatory cytokines, Toll-like receptor (TLR), Type I interferons (IFNs)
INTRODUCTION
Innate immune system is the first line barrier of host defense during pathogen infection, and is also critical for an effective adaptive immune system. Toll-like receptors (TLRs), the highly conserved type I transmembrane pattern recognition receptors (PRRs), are expressed on antigen presenting cells (APCs) such as macrophages and dendritic cells (DCs). There are 12 members of TLR family in mammals, and each TLR recognizes highly conserved structural motifs, known as pathogen-associated molecular patterns (PAMPs) from various pathogens (1, 2), or danger-associated molecular patterns (DAMPs) that are endogenous molecules released form necrotic or dying cells. The engagement of TLRs by PAMPs activates multiple signaling pathways to induce specific immune mediators, including inflammatory cytokines and type I interferons (IFNs), to eliminate the pathogens. Upon ligands binding to TLRs, TIR domain-containing adaptor proteins such as myeloid differentiation factor 88 (MyD88) and toll-interleukin 1 receptor (TIR) homology-domain-containing adaptor-inducing interferon-β (TRIF) are recruited to the TLR signaling complex. All TLRs, except TLR3, activate the MyD88-dependent pathway (3, 4). TLR-MyD88 signaling complex recruits the interleukin-1 receptor-associated kinase (IRAK) family, which results in activation of tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) (5). TRAF6, an E3 ubiquitin ligase, activates the downstream transducer molecule transforming growth factor β-activated kinase 1 (TAK1) complex via lysine (K)-63 chain ubiquitination (6). TAK1 mediates mitogen-activated protein kinases (MAPKs) and I kappa B kinase (IKK) activation, which lead to the activation of activator protein 1 (AP1) and nuclear factor-kappa B (NF-κB), respectively (4-6). TLR3 and TLR4 induce the TRIF-dependent pathway (3, 4). TLR-TRIF signaling complex recruits downstream adaptor molecules, including TRAF6, TRAF3, and receptor-interacting protein 1 (RIP1), which results in the activation of IFN regulatory factor 3 (IRF3), AP1, and NF-κB (4, 7, 8). Although these TLR signaling pathways are critical for host cell defense, uncontrolled or excessive activation of TLRs can also cause inflammatory host cell damage, such as autoimmune disease and chronic inflammation (1, 3). Thus, it is imperative that the TLR signaling pathways must be tightly controlled.
Glycogen synthase kinase 3 (GSK3) was originally identified as an enzyme that regulates glycogen metabolism (9). GSK3 is a highly conserved and ubiquitously expressed serine/threonine kinase. Mammalian GSK3 has two isoforms, GSK3α and GSK3β, encoded by two distinct genes, gsk3α and gsk3β. Although both the isoforms are structurally similar, they are not functionally identical (10, 11). GSK3 is involved in various cellular functions, including embryonic development, cell differentiation, cell proliferation and cell death (12-14). GSK3 is constitutively active under basal conditions, but inhibited by phosphoinositide 3-kinase (PI3K)-Akt or MAPKs pathway through serine phosphorylation (Ser21 for GSK3α and Ser9 for GSK3β) (15). In addition, tyrosine phosphorylation of GSK3 (Tyr279 for GSK3α and Tyr216 for GSK3β) promotes its activity (16). During the past decade, numerous studies have demonstrated the regulatory roles of GSK3β in innate immune responses, especially the TLR signaling pathways (17-19). Although GSK3 has been reported as a key mediator of MyD88-dependent cytokine production in TLR signaling against bacterial infections, little is known about the regulatory roles of GSK3 in viral infections. Recently, we demonstrated that GSK3β plays a role in TLR3-mediated pro-inflammatory cytokine production by promoting the TRIF-assembled signaling complex (20).
In this review, we first review the current knowledge about the involvement of GSK3 in TLR-mediated immune responses in innate immune cells. In addition, we will also focus on the regulatory role of GSK3β in TLR3-mediated immune response triggered by viral infections.
GSK3β IN TLR4 RESPONSES
TLR4, the first reported mammalian TLR, is the most well characterized PRR that mainly recognizes lipopolysaccharide (LPS) on the cell surface from Gram-negative bacteria (21, 22). TLR4 mediates both MyD88- and TRIF-dependent signaling pathways to produce inflammatory cytokines and type I IFNs (Fig. 1). The engagement of TLR4 by LPS activates the MyD88-dependent pathway through TIR domain containing adaptor protein (TIRAP), and then recruits the downstream IRAK family and TRAF6 (4, 5). TRAF6 interacts with TAK1, TAK1-binding protein 1 (TAB1) and TAB2, and catalyzes K63-linked ubiquitination, resulting in the phosphorylation and activation of TAK1 (6). Activated TAK1 phosphorylates the canonical IKK complex and MAPK, and thereby activates transcription factors NF-κB, CREB and AP1, which lead to the regulation of pro- and anti-inflammatory cytokine production (Fig. 1). Phosphoinositide 3-kinase (PI3K) is also involved in TLR4 signaling pathways (23). LPS stimulation further activates the PI3K-Akt pathway to regulate inflammatory genes (24).
Fig. 1. GSK3β in TLR4 responses. GSK3β transcriptionally regulates pro- and anti-inflammatory cytokines and IFN-β production in TLR4 signaling pathway. In basal condition, GSK3β is constitutively active. GSK3β inhibits the binding of CREB to CBP, whereas it promotes the binding of NF-κB to CBP, leading to reduced anti-inflammatory cytokine production and enhanced pro-inflammatory production, respectively. GSK3β also inhibits AP1, such as c-Jun and ATF2, leading to reduced IFN-β production. Upon increased PI3K activation, GSK3β is phosphorylated and inactivated, leading to enhanced anti-inflammatory cytokine and IFN-β production and reduced pro-inflammatory cytokine production.
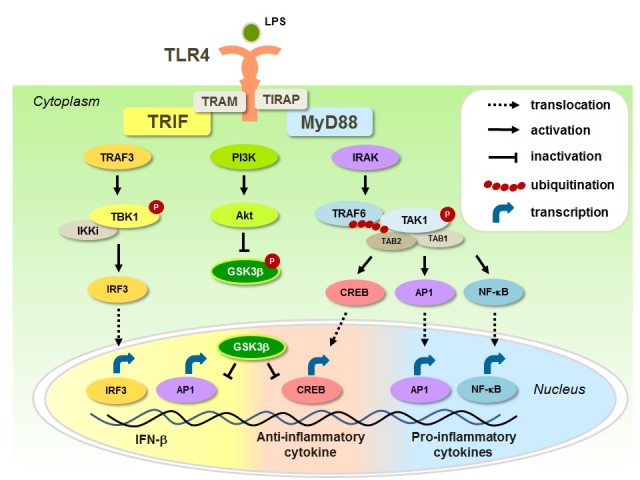
Several groups have reported that the activity of GSK3β regulates pro- and anti-inflammatory cytokine production in TLR4 responses. Martin et al. initially demonstrated the regulatory roles of GSK3β, which is involved in diverse TLR-mediated inflammatory cytokine production (25). LPS stimulation leads to the phosphorylation and inactivation of GSK3β through the PI3K-Akt-dependent pathway. In GSK3-inactivated human monocytes using pharmacological inhibitors or GSK3β-deficient mouse embryonic fibroblast (MEFs), the levels of pro-inflammatory cytokines, such as IL-1β, IL-6, IL-12 and TNF-α, were reduced, while the levels of the anti-inflammatory cytokine IL-10 were enhanced. Moreover, GSK3β modulated the transcriptional activity of cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB) to interact with nuclear coactivator CREB-binding protein (CBP). In addition, a recent publication has reported that the ability of GSK3β to control pro- and anti-inflammatory cytokine production is also regulated by the mammalian target of rapamycin complex 1 (mTORC1) pathway in LPS-stimulated human monocytes (26). Studies by Nandan et al. and Paul et al. further indicated that GSK3β could also regulate the IL-10 production via CREB against visceral leishmaniasis (VL) in murine macrophages and human macrophages (27, 28).
Apart from the above, it has been reported that GSK3β was involved in various signaling mechanisms for cytokine production. For example, GSK3β differentially regulated IL-1β production and anti-inflammatory cytokine IL-1 receptor antagonist (IL-1Ra) production, the endogenous IL-1β inhibitor. Inhibition of GSK3β activity or siRNA knockdown of GSK3β augmented LPS-induced IL-1Ra production through ras-related C3 botulinum toxin substrate 1 (Rac1)-ERK axis in human monocytes (29). GSK3β was also required for the synergistic action of IFN-γ on LPS-induced IL-6 production in murine macrophages (30). It has been reported that the priming of macrophages by IFN-γ, a macrophage activating factor, potentiates the TLR-induced cytokine production. GSK3β interacted with signaling transducer and activator of transcription 3 (STAT3) and positively regulated the activation of IFN-γ-induced STAT3. Moreover, recent studies demonstrated that GSK3β was required for the ubiquitination and stabilization of apoptosis signal-regulating kinase (ASK1), which is an important mediator in TLR-mediated inflammatory cytokine production (31). These data suggest that GSK3β as a potent mediator of inflammatory cytokine production in TLR4 signaling pathway.
TLR4 also utilizes the TRIF-dependent pathway through TRIF-related adaptor molecule (TRAM) to produce IFN-β. Studies by Wang et al. indicated that GSK3β negatively regulates the LPS-induced IFN-β production in vitro and in vivo (32). In both wild-type and MyD88-deficient macrophages, GSK3β phosphorylation was induced after LPS stimulation. While pharmacological inactivation or siRNA knockdown of GSK3β resulted in higher levels of IFN-β, forced expression of kinase-dead GSK3β resulted in lower levels of IFN-β in response to LPS. Interestingly, GSK3β modulated the LPS-induced nuclear levels of c-Jun, but not NF-κB or IRF3, to regulate IFN-β production. Together, these studies suggest that GSK3β is necessary for TLR4-mediated inflammatory response.
GSK3 AND TLR2 SIGNALING
TLR2 is expressed on cell surface and forms heterodimers with TLR1 or TLR6. In general, TLR2 recognizes bacterial cell wall components, including peptidoglycan (PGN), lipoteichoic acid (LTA) and lipoprotein; TIRAP and MyD88 participated in signal transduction to produce pro- and anti-inflammatory cytokines (33-35).
Studies by Martin et al. revealed the involvement of GSK3 in TLR2-mediated cytokine production (25). Pharmacological inhibition of GSK3 resulted in higher levels of IL-1β and Il-12p40 production, but lower levels of IL-10 production, in response to LTA. Subsequently, the regulatory role of GSK3β in IFN-γ and TLR2-mediated IL-10 production in human macrophages was also demonstrated (36). IFN-γ enhanced TLR2-mediated GSK3 activity through PI3K-Akt pathway, and GSK3 suppressed the IL-10 production by regulating transcriptional activity and expression of AP1. Zhang et al. investigated that the regulatory role of GSK3β in Francisella tularensis infection in murine macrophages related to TLR2 signaling pathway (37). PI3K-Akt-GSK3β axis differentially modulated pro- and anti-inflammatory cytokine production through TLR2-mediated NF-κB and CREB activity in response to F. tularensis live vaccine strain (LSV) stimulation. Similarly, GSK3β regulated the TLR2-mediated inflammatory response in murine microglial cells against Staphylococcus aureus infection (38). Inhibition of GSK3β by pharmacological inhibitors reduced TNF-α production and inducible NO synthase (iNOS) synthesis through regulating the NF-κB activity. Interestingly, recent studies showed that GSK3α and β isoforms differentially regulated S. aureus-induced IL12-p40 expression in endothelial cells (39). In stimulation of PGN from S. aureus, both isoforms of GSK3 were phosphorylated through the TLR2-PI3K-Akt pathway. The levels of IL-12p40 were elevated in GSK3α knockdown, while they were reduced in GSK3β knockdown. Together, these studies suggest that GSK3 is required for TLR2-dependent immune response.
GSK3β IN TLR3 SIGNALING
TLR3 recognizes the double-stranded RNA (dsRNA), an intermediate produced during the replication of many RNA viruses (40-42). TLR3 activation triggers signaling pathways through the sole adaptor TRIF to produce type I IFN and pro-inflammatory cytokines (Fig. 2). It has been reported that the TLR3-mediated IFN-β production was impaired in the TRIF knockout mice, suggesting that TRIF is essential for TLR3-mediated signaling pathway (43). The TLR3-TRIF signaling complex recruits downstream adaptor molecules, including TRAF3, TRAF6 and RIP1, which lead to the activation of transcription factors, such as IRF3, AP1 and NF-κB (Fig. 2). It has been reported that TRAF3 is a critical linker between TRIF and tank-binding kinase 1 (TBK1)-IKKi complex (7). It has been known that TBK1 directly phosphorylates IRF3, which enhances its dimerization and nuclear translocation, resulting in the production of IFN-β (42, 44, 45). RIP1 and TRAF6 are also recruited to TRIF and further interact with downstream TAK1, which subsequently activates AP1 and NF-κB, through MAPKs and IKKα-IKKβ complex, respectively (46, 47). While many studies have suggested the regulatory roles of GSK3β in TLR signaling, it is poorly understood how GSK3β regulates TLR3 signaling.
Fig. 2. GSK3β in TLR3 signaling. GSK3β positively regulates both pro-inflammatory cytokines and IFN-β production in TLR3 signaling pathway. Upon TLR3 activation, GSK3β interacts with TRAF6-TAK1 and undergoes K63-linked ubiquitination, and further promotes AP1, such as c-Fos, leading to pro-inflammatory cytokine production. GSK3β interacts with TRAF3-TBK1 complex and enhances TBK1 phosphorylation, thereby activating IRF3, leading to IFN-β production.
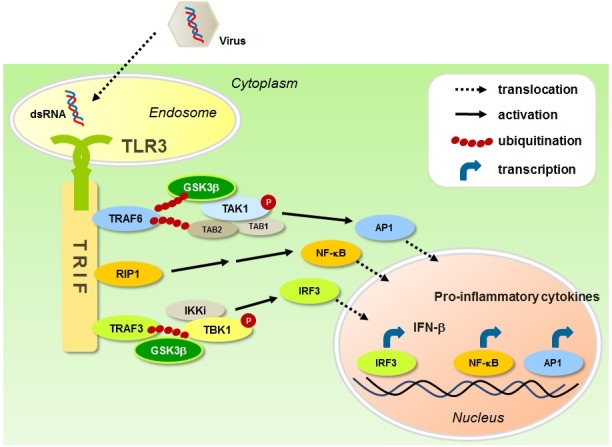
Retinoic acid-inducible gene 1-like receptor (RLR)-mediated signaling pathway shares a number of components in common with TLR-mediated signaling pathways (48, 49). Studies by Lei et al. showed that GSK3β positively regulated the RLR-mediated IFN-β induction at the TBK1 level (50). GSK3β, but not GSK3α, modulated the virus-triggered TBK1 autophosphorylation and self-association, which lead to the IRF3 activation and IFN-β induction independently of the kinase activity of GSK3β. Consistently, our previous studies demonstrated that GSK3β interacted with TRAF3 and positively regulated TLR3-mediated IFN-β production (20). The expression levels of IFN-β were reduced in GSK3β knockdown murine macrophages or Gsk3b−/− MEFs in response to poly I:C stimulation. Moreover, the poly I:C-induced IRF3 phosphorylation, nuclear translocation and dimerization, and TBK1 phosphorylation were reduced in Gsk3b−/− MEFs compared with control cells. In contrast, neither GSK3 inhibition with SB216763 or knockdown of GSK3α affected the IFN-β expression in murine macrophages. These two studies suggest that GSK3 is required for both TLR3- and RLR-induced antiviral response, and the effects of GSK3β on IFN-β production is independent of its kinase activity.
Although the mechanisms of TLR3 signaling leading to type I IFNs have been well identified, the mechanisms of inflammatory cytokine production were largely unknown. Recently, we demonstrated that GSK3β had a positive role in regulating the pro-inflammatory cytokine production in TLR3 signaling (20). Knockdown of GSK3β, but not GSK3α or inhibition of its kinase activity, significantly reduced the poly I:C-induced pro-inflammatory cytokine production in murine macrophages. GSK3β positively regulated the poly I:C-induced ERK and p38 phosphorylation, and thereby enhanced the c-Fos expression. Interestingly, GSK3β interacted with TRAF6 but not with RIP1, and underwent K63-linked ubiquitination at K183 by TRAF6. Moreover, GSK3β ubiquitination potentiated the formation of the TRIF-mediated signaling complex. These results suggested that GSK3β controls the TRAF6-TAK1-MAPK axis to regulate pro-inflammatory cytokine production, and the TRAF3-TBK1-IRF3 axis to regulate IFN-β production. Together, these studies suggest that GSK3β acts as a key regulator in TLR3-mediated antiviral responses.
CONCLUDING REMARKS
During the past decades, numerous studies have indicated a role for GSK3β in TLR-mediated inflammatory response. In general, inhibition of GSK3β differentially controls pro- and anti-inflammatory cytokine production by regulating the CREB activity in MyD88-mediated TLR signaling pathways such as TLR2 and TLR4. In addition, GSK3 kinase activity is required for its role in inflammatory cytokine production. Whereas most studies of GSK3β function have focused on its role in MyD88-mediated signaling pathways during bacterial infection, recent studies have demonstrated the roles of GSK3β in TRIF-mediated antiviral responses. It has been reported that GSK3β negatively regulates TLR4-mediated IFN-β production through transcription factor c-Jun. In contrast, our recent study has demonstrated that GSK3β interacts with TRAF3 and acts as a positive regulator in TLR3-mediated IFN-β production through the TRAF3-TBK1-IRF3 axis.
Interestingly, we also demonstrated that GSK3β interacts with TRAF6, and its K63-linked ubiquitination by TRAF6 positively regulates the TLR3-mediated pro-inflammatory cytokine production through TRAF6-TAK1-MAPK axis. Although further studies are needed to clarify the details about the regulatory mechanisms of TLRs signaling by GSK3β, it is important that GSK3β has an ability to selectively regulate both MyD88- and TRIF-dependent pathways, suggesting that GSK3β has a potential therapeutic target to treat several inflammatory states.
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2015R1A6A3A0102 0489) (to R.K.) and by the Ministry of Science, ICT and Future Planning (No. 2013R1A2A1A05005153; No. 2012R1A5A10 48236) (to S.Y.L).
References
- 1.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. (1997);91:295–268. doi: 10.1016/S0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. (2004);4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. (2006);124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. (2010);11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 5.Kawagoe T, Sato S, Matsushita K, et al. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol. (2008);9:684–691. doi: 10.1038/ni.1606. [DOI] [PubMed] [Google Scholar]
- 6.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. (2009);458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 7.Oganesyan G, Saha SK, Guo B, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. (2006);439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 8.Meylan E, Burns K, Hofmann K, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. (2004);5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 9.Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. (1980);107:519–527. doi: 10.1111/j.1432-1033.1980.tb06059.x. [DOI] [PubMed] [Google Scholar]
- 10.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. (1990);9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Force T, Woodgett JR. Unique and overlapping functions of GSK-3 isoforms in cell differentiation and proliferation and cardiovascular development. J Biol Chem. (2009);284:9643–9647. doi: 10.1074/jbc.R800077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. (2003);116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. (2004);29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. (2009);156:885–898. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross DA, Watt PW, Shaw M, et al. Insulin activates protein kinase B, inhibits glycogen synthase kinase-3 and activates glycogen synthase by rapamycin-insensitive pathways in skeletal muscle and adipose tissue. FEBS Lett. (1997);406:211–215. doi: 10.1016/S0014-5793(97)00240-8. [DOI] [PubMed] [Google Scholar]
- 16.Bax B, Carter PS, Lewis C, et al. The structure of phosphorylated GSK-3beta complexed with a peptide, FRATtide, that inhibits beta-catenin phosphorylation. Structure. (2001);9:1143–1152. doi: 10.1016/S0969-2126(01)00679-7. [DOI] [PubMed] [Google Scholar]
- 17.Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. (2010);31:24–31. doi: 10.1016/j.it.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Brown J, Martin M. Glycogen synthase kinase 3: a point of convergence for the host inflammatory response. Cytokine. (2011);53:130–140. doi: 10.1016/j.cyto.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Kumar A, Lamont RJ, Scott DA. GSK3β and the control of infectious bacterial diseases. Trends Microbiol. (2014);22:208–217. doi: 10.1016/j.tim.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko R, Park JH, Ha H, Choi Y, Lee SY. Glycogen synthase kinase 3β ubiquitination by TRAF6 regulates TLR3-mediated pro-inflammatory cytokine production. Nat Commun. (2015);6:6765. doi: 10.1038/ncomms7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. (1997);388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 22.Poltorak A, He X, Smirnova I. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. (1998);282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 23.Arbibe L, Mira JP, Teusch N, et al. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. (2000);1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 24.Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. (2002);277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 25.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. (2005);6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Brown J, Gu Z, et al. Convergence of the mammalian target of rapamycin complex 1- and glycogen synthase kinase 3-β-signaling pathways regulates the innate inflammatory response. J Immunol. (2011);186:5217–5226. doi: 10.4049/jimmunol.1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nandan D, Camargo de Oliveira C, Moeenrezakhanlou A, et al. Myeloid cell IL-10 production in response to leishmania involves inactivation of glycogen synthase kinase-3β downstream of phosphatidylinositol-3 kinase. J Immunol. (2012);188:367–378. doi: 10.4049/jimmunol.1100076. [DOI] [PubMed] [Google Scholar]
- 28.Paul J, Naskar K, Chowdhury S, Chakraborti T, De T. TLR mediated GSK3β activation suppresses CREB mediated IL-10 production to induce a protective immune response against murine visceral leishmaniasis. Biochimie. (2014);107:235–246. doi: 10.1016/j.biochi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Rehani K, Wang H, Garcia CA, Kinane DF, Martin M. Toll-like receptor-mediated production of IL-1Ra is negatively regulated by GSK3 via the MAPK ERK1/2. J Immunol. (2009);182:547–553. doi: 10.4049/jimmunol.182.1.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beurel E, Jope RS. GSK3 promotes the synergistic action of interferon-gamma on lipopolysaccharide-induced IL-6 production in RAW264.7 cells. Cell Signal. (2009);21:978–985. doi: 10.1016/j.cellsig.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noh KT, Park YM, Cho SG, Choi EJ. GSK-3β-induced ASK1 stabilization is crucial in LPS-induced endotoxin shock. Exp Cell Res. (2011);317:1663–1668. doi: 10.1016/j.yexcr.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Garcia CA, Rehani K, et al. IFN-beta production by TLR4-stimulated innate immune cells is negatively regulated by GSK3-beta. J Immunol. (2008);181:6797–6892. doi: 10.4049/jimmunol.181.10.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozinsky A, Underhill DM, Fontenot JD, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc Natl Acad Sci U S A. (2000);97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farhat K, Riekenberg S, Heine H. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J Leukoc Biol. (2008);83:692–701. doi: 10.1189/jlb.0807586. [DOI] [PubMed] [Google Scholar]
- 35.Borrello S, Nicolò C, Delogu G, Pandolfi F, Ria F. TLR2: a crossroads between infections and autoimmunity? Int J Immunopathol Pharmacol. (2011);24:549–556. doi: 10.1177/039463201102400301. [DOI] [PubMed] [Google Scholar]
- 36.Hu X, Paik PK, Chen J, et al. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity. (2006);24:563–574. doi: 10.1016/j.immuni.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Zhang P, Katz J, Michalek SM. Glycogen synthase kinase-3beta (GSK3beta) inhibition suppresses the inflammatory response to Francisella infection and protects against tularemia in mice. Mol Immunol. (2009);46:677–687. doi: 10.1016/j.molimm.2008.08.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng YL, Wang CY, Huang WC, et al. Staphylococcus aureus induces microglial inflammation via a glycogen synthase kinase 3beta-regulated pathway. Infect Immun. (2009);77:4002–4008. doi: 10.1128/IAI.00176-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cortés-Vieyra R, Silva-García O, Oviedo-Boyso, et al. The Glycogen Synthase Kinase 3α and β Isoforms Differentially Regulates Interleukin-12p40 Expression in Endothelial Cells Stimulated with Peptidoglycan from Staphylococcus aureus. PLoS One. (2015);10:e0132867. doi: 10.1371/journal.pone.0132867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. (2001);413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto M, Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C). Adv Drug Deliv Rev. (2008);60:805–812. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Perales-Linares R, Navas-Martin S. Toll-like receptor 3 in viral pathogenesis: friend or foe? Immunology. (2013);140:153–167. doi: 10.1111/imm.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto M, Sato S, Hemmi H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. (2003);301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 44.Fitzgerald KA, McWhirter SM, Faia KL, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. (2003);4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 45.Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem. (2007);282:15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- 46.Sato S, Sugiyama M, Yamamoto M, et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol. (2003);171:4304–4310. doi: 10.4049/jimmunol.171.8.4304. [DOI] [PubMed] [Google Scholar]
- 47.Meylan E, Burns K, Hofmann K, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. (2004);5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 48.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. (2009);21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chattopadhyay S, Sen GC. dsRNA-activation of TLR3 and RLR signaling: gene induction-dependent and independent effects. J Interferon Cytokine Res. (2014);34:427–436. doi: 10.1089/jir.2014.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lei CQ, Zhong B, Zhang Y, et al. Glycogen synthase kinase 3β regulates IRF3 transcription factor-mediated antiviral response via activation of the kinase TBK1. Immunity. (2010);33:878–889. doi: 10.1016/j.immuni.2010.11.021. [DOI] [PubMed] [Google Scholar]


