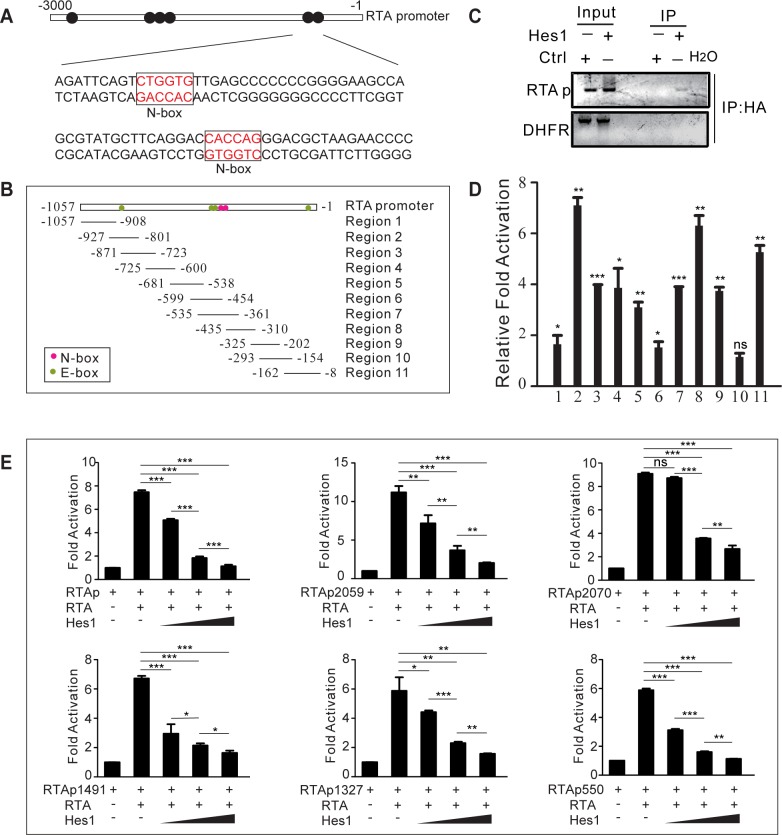
Fig 5. Hes1 inhibits RTA self-transactivation.
(A) Schematic illustrating the N-box Hes1 binding motifs within the RTA promoter. (B) Primers were designed to cover the RTA promoter. (C, D) 293.219 cells plated in 100 mm dish at 4 million cells 24 h before transfection. HA-Hes1 and control plasmids (12 μg) were transfected and ChIP assay was performed 24 h after transfection. Extracts were subjected to immunoprecipitation with an anti-HA-tag antibody and purified DNA elute was quantified by gel analysis (C) or qPCR with the indicated primers from B (D). (E) Hes1 represses the transcriptional activity of the RTA promoter. Dual luciferase assay was performed in HEK293T cells plated in 12-well plates at 0.5 million per well 24 h after transfection. The cells were transiently transfected with expression vectors containing RTA (1 μg), reporter plasmids containing full length RTA promoter or RTA promoter truncations (100 ng), and increasing amounts of Hes1 (250 ng, 500 ng or 1μg) using Lipofectamine 2000. Total transfected DNA was normalized with pcDNA3.1. Data were expressed as the mean ± s.e.m., n = 3, *p<0.05, **p<0.01, ***p<0.001.