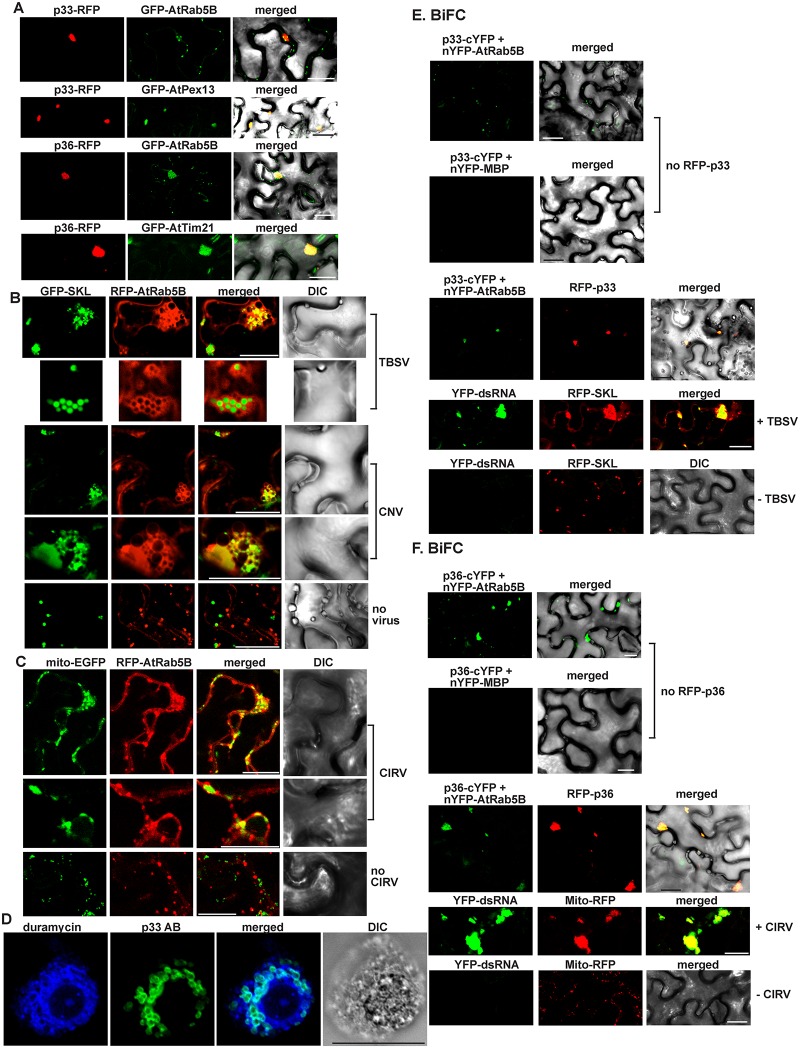
Fig 2. Recruitment of Arabidopsis Rab5 into the tombusvirus replication compartment in N. benthamiana.
(A) Confocal laser microscopy shows partial co-localization of TBSV RFP-tagged p33 replication protein or CIRV RFP-tagged p36 with the GFP-AtRab5B protein in N. benthamiana cells. Expression of the above proteins from the 35S promoter was achieved after agro-infiltration into N. benthamiana leaves. Scale bars represent 20 μm. (B) Partial re-localization of RFP-AtRab5B protein to the peroxisomes (marked by GFP-SKL) in N. benthamiana cells infected with either TBSV or CNV. The bottom image shows the absence of re-localization of RFP-AtRab5B protein to the peroxisome in the mock-infected plant leaves. Scale bars represent 20 μm. (C) Partial re-localization of RFP-AtRab5B protein to the mitochondria (marked by mito-EGFP) in N. benthamiana cells infected with CIRV. The bottom image shows the absence of re-localization of RFP-AtRab5B protein to the mitochondria in the mock-infected plant leaves. Scale bars represent 20 μm. (D) TBSV infection induces membrane proliferation, which is occasionally visualized as aggregated circle-like structures. These membranous structures are enriched in PE in plant cells. The confocal laser microscopy image shows the enrichment of PE and its co-localization with the TBSV p33/p92 replication proteins, which were detected with p33-specific primary antibody and secondary antibody conjugated with Alexa Fluor488. Localization of PE is detected by using biotinylated duramycin peptide and streptavidin conjugated with Alexa Fluor 405. DIC images are shown on the right. Scale bars represent 20 μm. (E) Top image: In planta interaction between TBSV p33-cYFP replication protein and the nYFP-AtRab5B protein. Expression of the above proteins from the 35S promoter was done after agro-infiltration into N. benthamiana leaves. Note that p33-cYFP and the nYFP-AtRab5B protein were detected by bimolecular fluorescence complementation (BiFC). Control BiFC experiments included nYFP-MBP protein. Bottom images: The interaction between p33 replication protein and AtRab5B occurs in the replication compartment decorated by RFP-p33. As expected, the enlarged replication compartment (highlighted via RFP-SKL) also contained the viral dsRNA replication intermediate only in TBSV-infected cells (second panel form the bottom) but not in the mock-inoculated cells (bottom panel). Scale bars represent 20 μm. (F) The corresponding experiments with the CIRV p36 protein and AtRab5B (see panel E for details). Scale bars represent 20 μm.