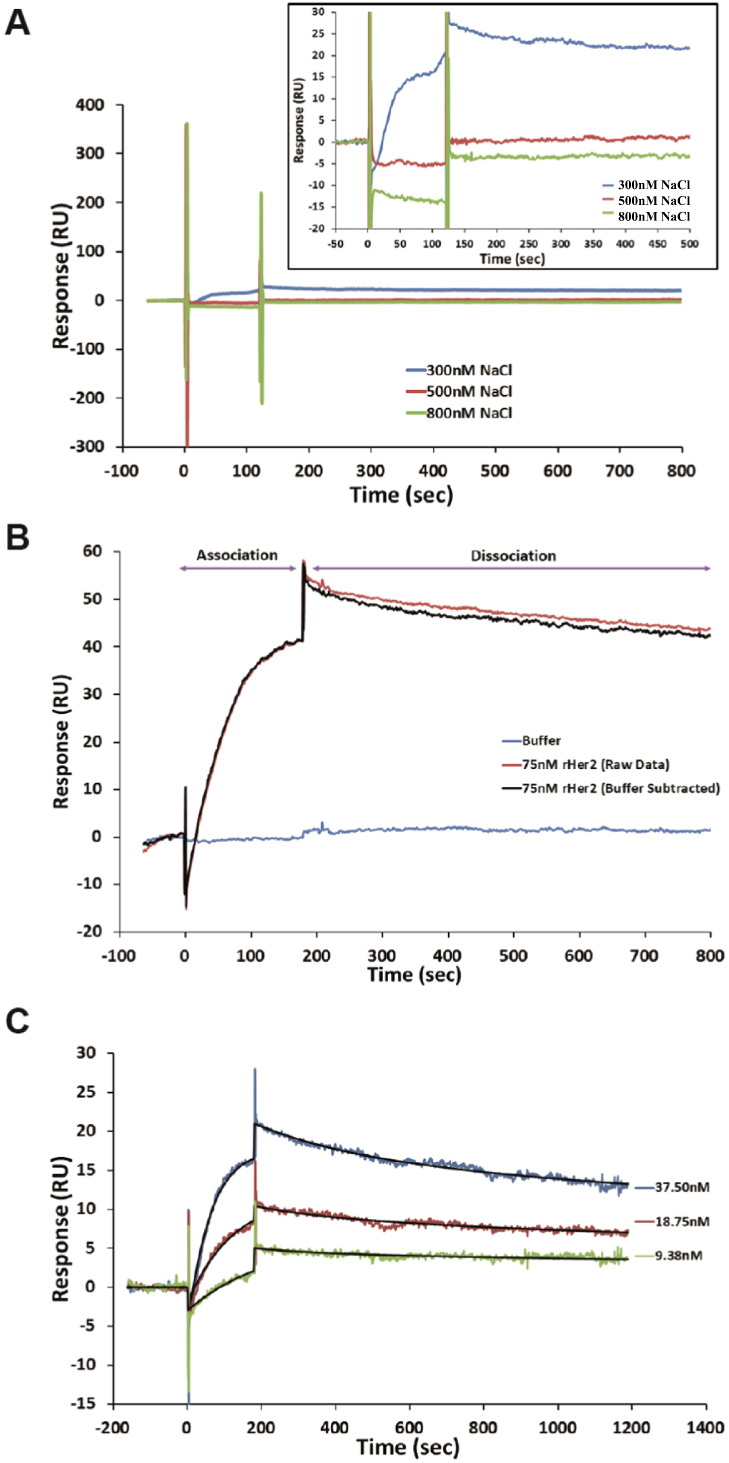
Fig. 1.
Analysis of the interaction between EPO and rHER2 on Biacore
The analysis was carried out on immobilized EPO. (A) Double reference subtracted binding of 75 nM rHER2 to EPO was performed over a range of salt concentrations (300–800 mM). The binding of rHER2 was diminished between 300 and 500 mM NaCl suggesting the importance of charge for the interaction. The overlay box shows an expansion of each sample. In (B) the binding profile for rHER2 in HBS-EP+ is shown. The red trace is the 75 nm rHER2 and the blue trace 0 nM (buffer only) raw data (online reference cell subtracted). The black trace is the double reference subtracted data (75 nM rHER2 minus 0 nM response). (C) Kinetic analysis of three HER2 concentrations (double reference subtracted data shown) is fitted with a bivalent analyte model (black line) with local Rmax fitting. The kinetic evaluation suggested ka1 = 1.29 × 105 M− 1 s− 1, kd1 = 2.03 × 10− 3 s− 1.