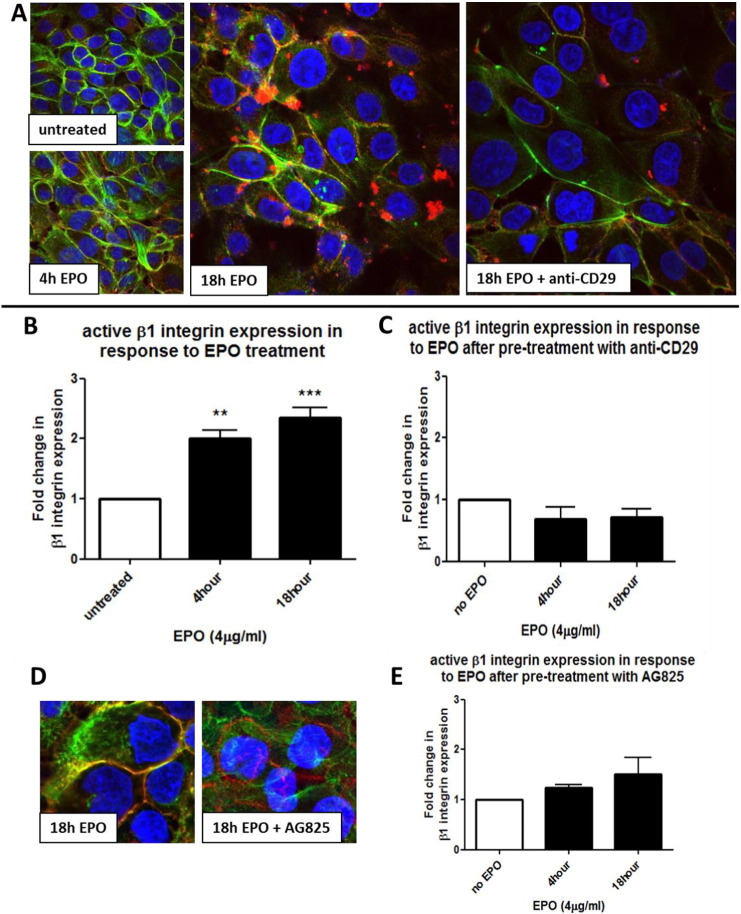
Fig. 4.
EPO induces a pHER2-dependent activation of β1 integrin
(A, D) 16HBE14o cells were grown on coverslips and treated with EPO (4 μg/ml) for various times or left untreated (as indicated). Some wells were pre-treated with a specific anti-β1-integrin neutralizing antibody (anti-CD29, 1 μg/ml, 2 h) or pretreated with a HER2 tyrosine kinase inhibitor (AG825, 10 μM, 2 h), as indicated on images.
β1-integrin (red) was stained with a mouse integrin-β1 antibody (JB1B, Santa Cruz) and a Texas Red goat anti-mouse IgG secondary antibody. β1-integrin activation is indicated by clustering and co-localization with F-actin fibers, stained with Phalloidin (green), resulting in the orange clusters visible at 18 h EPO treatment (A). In (B) cells were treated with EPO (4 μg/ml) for varying time points, cellular protein was subjected to Western blot analysis and probed for active β1-integrin and against ERK2 for normalization. In (C) cells were first pre-treated with anti-CD29 (1 μg/ml, 2 h) and then exposed to EPO (4 μg/ml) for varying time points. Cellular protein was subjected to Western blot analysis and probed for active β1-integrin and against ERK2 for normalization. In (D) confocal microscopy images show the comparison of cells treated with EPO for 18 h in the absence or presence of AG825 (as indicated). β1-integrin (red) activation is indicated by clustering and co-localization with F-actin fibers. In (E) 16HBE14o cells were first pre-treated with AG825 (10 μM, 2 h) and then exposed to EPO (4 μg/ml) for varying time points. The graphs show the fold change in active β1-integrin expression levels in response to EPO treatment compared to untreated cells in the absence (B) or presence (C, E) of inhibitors at the indicated times. (n = 3, mean ± sem; **p < 0.01, ***p < 0.001).