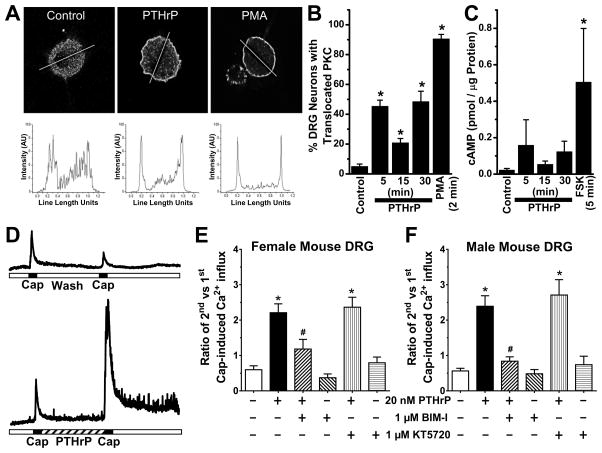
Figure 6.
PTHrP exposure of mouse sensory neurons leads to PKC activation, and is critical for potentiation of TRPV1 activity. A, Representative confocal microscopic images (top row) of cultured mouse DRG neurons, with or without the treatment of PTHrP (10 nM, 2 min) or PMA (100 nM, 2 min), immunostained with anti-PKCε antibody. Traces in the bottom row depict PKCε distribution profiles across the respective drawn line determined using NIH Image J. B, Quantification of percentage of cultured mouse DRG neurons with PKCε translocation (n = >100 cells in each group obtained from ≥3 batches of cultures). C, cAMP ELISA experiments showed no significant increase in cAMP levels in mouse DRG neurons treated with PTHrP (10 nM). Forskolin (10 μM, 5 min) significantly increased cellular cAMP levels compared to untreated control DRG neurons. D, Representative traces of Ca2+ influx in cultured mouse DRG neurons, in response to two successive capsaicin applications (50 nM, 15 sec), with or without PTHrP treatment (20 nM, 5 min) in between. E–F, Quantification of the ratio of 2nd vs 1st capsaicin-induced Ca2+ influx, under control and 20 nM PTHrP treatment conditions. Neurons were treated with inhibitors of PKC (BIM-I; 1 μM), PKA (KT5720; 1 μM) and Src (PP2; 100 nM) before the 1st capsaicin application and continued throughout PTHrP or control buffer applications. Inhibition of PKC, but not Src or PKA attenuated PTHrP-sensitization of capsaicin-induced Ca2+ influx in cultured DRG neurons from both female (E) and male (F) mice. Data are presented as mean ± SEM (n = 11–52 neurons in each group). *p<0.05 vs untreated control (One way ANOVA with Dunnett’s post hoc correction), and #p<0.05 vs PTHrP group in panels E–F (Unpaired Student’s T-test).