Abstract
Objectives
Sub-anesthetic doses of ketamine have been found to provide rapid antidepressant actions, indicating that the cellular signaling systems targeted by ketamine are potential sites for therapeutic intervention. Ketamine acts as an antagonist of N-methyl-D-aspartate (NMDA) receptors, and animal studies indicate that subsequent augmentation of signaling by α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors is critical for the antidepressant outcome.
Methods
In this study, we tested if the inhibitory effect of ketamine on glycogen synthase kinase-3 (GSK3) affected hippocampal cell-surface AMPA receptors using immunoblotting of membrane and synaptosomal extracts from wild-type and GSK3 knockin mice.
Results
Treatment with an antidepressant dose of ketamine increased the hippocampal membrane level of the AMPA glutamate receptor (GluA)1 subunit, but did not alter the localization of GluA2, GluA3, or GluA4. This effect of ketamine was abrogated in GSK3 knockin mice expressing mutant GSK3 that cannot be inhibited by ketamine, demonstrating that ketamine-induced inhibition of GSK3 is necessary for up-regulation of cell surface AMPA GluA1 subunits. AMPA receptor trafficking is regulated by post-synaptic density-95 (PSD-95), a substrate for GSK3. Ketamine treatment decreased the hippocampal membrane level of phosphorylated PSD-95 on Thr-19, the target of GSK3 that promotes AMPA receptor internalization.
Conclusions
These results demonstrate that ketamine-induced inhibition of GSK3 causes reduced phosphorylation of PSD-95, diminishing the internalization of AMPA GluA1 subunits to allow for augmented signaling through AMPA receptors following ketamine treatment.
Keywords: AMPA, depression, glycogen synthase kinase-3, GSK3, ketamine, PSD-95
Major depressive disorder is a prevalent, progressive, and debilitating disease (1). Current treatments for major depression are inadequate because they take weeks to become effective, and they are often ineffective or not tolerated (2). Thus, there is a great need for new interventions that act rapidly, provide sustained antidepressant actions, and are therapeutic in a greater portion of patients. The N-methyl-D-aspartate (NMDA) receptor antagonist ketamine fulfills some of these requirements. Administration of ketamine produces a rapid antidepressant action that is evident in a time-frame of hours instead of weeks, and ketamine is generally well-tolerated and is effective in many depressed patients who do not respond to classical antidepressants (3–5). On the other hand, the antidepressant action of ketamine is transient, usually being effective for only one to two weeks, and ketamine faces significant hurdles to become widely used as a maintenance regimen because of the risk for adverse effects and potential for abuse (5, 6). Thus, the discovery of the rapid and effective antidepressant action of ketamine represents a crucial advance in the treatment of depression, but clarification of its antidepressant mechanism of action is needed to develop alternative treatments that are longer-lasting and better tolerated.
The antidepressant mechanism of action of ketamine is not clear, although most findings indicate that it is likely to involve downstream signaling outcomes of blocking the NMDA receptor (4, 7). Several important findings have begun to identify mechanisms contributing to ketamine's antidepressant effect, including increasing brain-derived neurotrophic factor (BDNF) levels (8) and modulation of mTOR signaling that is associated with synaptic modifications (9, 10). Furthermore, we previously found that ketamine's antidepressant effect is linked to inhibition of glycogen synthase kinase-3 (GSK3) (11). GSK3 is a feasible target for antidepressant effects because the two isoforms of GSK3, GSK3α and GSK3β, modulate many aspects of neuronal function, such as gene expression, neurogenesis, synaptic plasticity, and neuronal structure (12). GSK3 is partially active in unstimulated cells and it is regulated predominantly by inhibitory phosphorylation on an N-terminal serine residue, serine-21 of GSK3α and serine-9 of GSK3β. The functional effects of inhibitory serine-phosphorylation of GSK3 can be studied by using GSK3α21A/21A/β9A/9A knockin mice (13, 14). In these mice the regulatory serines of both GSK3 isoforms are mutated to alanines and this prevents the serine-phosphorylation and inhibition of GSK3. These mutations maintain GSK3 maximally active, but importantly within the physiological range since both GSK3 isoforms are expressed at normal levels.
Numerous links between GSK3 and depression have been reviewed (15) that suggest abnormally active GSK3 contributes to susceptibility to depression and inhibition of GSK3 is antidepressive, an action that may contribute to ketamine's antidepressant effect. For example, GSK3 is normally inhibited by neuromodulators that may be deficient in mood disorders (e.g., BDNF, serotonin), and the deficient inhibition of GSK3 can be counteracted by the mood stabilizer lithium (16), and by classical antidepressant monoamine reuptake inhibitors (17) that inhibit GSK3. We previously found that ketamine antidepressant treatment inhibits GSK3 in mouse hippocampus and cerebral cortex and that this is required for the rapid antidepressant action of ketamine in the learned helplessness model of depression-like behavior because it is blocked in GSK3 knockin mice (11). These results indicate that ketamine-induced inhibition of GSK3 is necessary for this antidepressant action of ketamine. Ketamine was also found to induce inhibition of GSK3 in lymphocytes of human depressed subjects (18). Furthermore, inhibitors of GSK3 enhanced the antidepressant effects of a subthreshold dose of ketamine in mice, suggesting this may provide a mechanism to reduce the deleterious characteristics of ketamine administration (19, 20).
The antidepressant effect of ketamine is also dependent on the activation of glutamatergic α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors. AMPA receptors are composed of glutamate receptor (GluA)1, GluA2, GluA3, and GluA4 subunits, most commonly a dimer of one subunit associated with a dimer of another type (21). AMPA receptor antagonist 2,3 dihydroxy-6-nitro-7-sulfamoyl benzo[f]quinoxaline-2,3-dione (NBQX) administration blocked the antidepressant effect of ketamine in several rodent models of depression-like behavior (8, 22, 23). Furthermore, inhibition of GSK3 reduces internalization of AMPA receptors and the antidepressant effects of the GSK3 inhibitor lithium depends on increased AMPA receptor-mediated signaling (24–26). GSK3 also phosphorylates the post-synaptic density-95 (PSD-95) protein, which regulates AMPA receptor trafficking (27). These interactions raise the possibility that ketamine increases cell surface AMPA receptors by its inhibitory effect on GSK3 modulating PSD-95, which was investigated in this study.
Material and methods
Mice
Male and female adult (7–12 weeks old) homozygous GSK3α/β21A/21A/9A/9A knockin mice and matched wild-type mice were used. Mice were housed in groups of 3–5 in standard cages in light and temperature controlled rooms and were treated in accordance with NIH and the University of Miami Institutional Animal Care and Use Committee regulations. Mice were treated intraperitoneally (i.p.) with saline or ketamine (10 mg/kg).
Western blotting
Isolated hippocampi were lysed in 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 10% glycerol, 1 μg/mL of leupeptin, aprotinin, and pepstatin A, 1 mM orthovanadate, 50 mM NaF, 0.1 μM okadaic acid, and 1 mM PMSF. Membrane and soluble fractions were separated using a membrane extraction kit (Calbiochem). Crude synaptosome fractions were prepared as described (9). Briefly, one hippocampus was homogenized in buffer containing 0.32 M sucrose, 20 mM HEPES, pH 7.4, 1 mM EDTA, 1 μg/mL of leupeptin, aprotinin, and pepstatin A, 1 mM orthovanadate, 50 mM NaF, 0.1 μM okadaic acid, and 1 mM PMSF and centrifuged for 10 min at 2,800 rpm at 4 C. The supernatant was further centrifuged at 12,000 rpm for 10 min and the pellet was resuspended and sonicated in protein lysis buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Tritom X-100, 0.1% SDS, 2 mM EDTA, 1 μg/mL of leupeptin, aprotinin, and pepstatin A, 1 mM orthovanadate, 50 mM NaF, 0.1 μM okadaic acid, and 1 mM PMSF.
Proteins were resolved by SDS-PAGE and nitrocellulose membranes were immunoblotted with antibodies to phospho-Ser21 GSK3α (#9316), phospho-Ser9 GSK3β (#9336), GluA1 (#13185), GluA2 (#5306), GluA3 (#4676), GluA4 (#8070), NMDA receptor (NR)1 (#5704), NR2 (#4205), PSD-95 (#3450) (Cell Signaling Technology, Danvers, MA, USA), total GSK3α/β (#05-412) (Millipore, Lake Placid, NY, USA), EGFR (#E12020) (BD Pharmingen, San Diego, CA, USA), phospho-Thr19 PSD-95 (#ab172628) (Abcam) and β-actin (#A5441) (Sigma, St Louis, MO, USA). EGFR is a membrane protein that has not been shown to be increased after a 30 min ketamine treatment and therefore was used as a loading control.
Multiplex analysis
The levels of phospho-mTOR and mTOR were assessed in hippocampal synaptosomal proteins using the milliplex map kit according to the manufacturer's instruction. Similarly, BDNF was assessed in hippocampal extracts using the mouse pituitary magnetic bead panel, milliplex map kit according to the manufacturer's instructions.
Statistical analysis
All data are means ± SEM. We used a two-way ANOVA, followed by a Bonferroni post hoc test to analyze the data and results were considered significant at p < 0.05.
Results
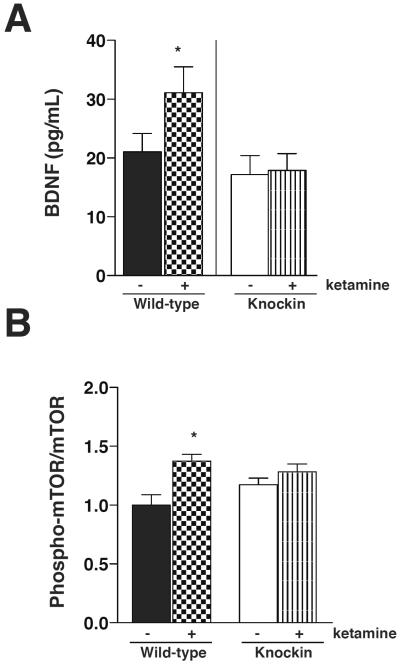
Several cellular mechanisms have been implicated in the antidepressant effect of ketamine. Among them, inhibition of GSK3, activation of the mTOR pathway and increased BDNF have been reported. Also, it is now well-established that GSK3 inhibits BDNF production and the mTOR pathway. We first tested if ketamine treatment, by increasing GSK3 inhibition, promotes BDNF and the mTOR pathway. To study the involvement of GSK3 in the ketamine response we used GSK3 knockin mice, where GSK3 is constitutively active. We found that the hippocampal BDNF level increased 24 h after treatment with 10 mg/kg ketamine and this was abolished in the GSK3 knockin mice, demonstrating that ketamine-induced up-regulation of BDNF is dependent on ketamine inhibiting GSK3 (Fig. 1A). Similarly the increase of the hippocampal synaptosomal phospho-mTOR level after a 30 min treatment with ketamine (10 mg/kg) was attenuated in the GSK3 knockin mice (Fig. 1B). Altogether, we found that GSK3 inhibition contributes to BDNF production and mTOR activation after ketamine treatment, suggesting that GSK3 acts early in the response to ketamine.
Fig. 1.
Glycogen synthase kinase-3 (GSK3) inhibits brain-derived neurotrophic factor (BDNF) production and mTOR activation after ketamine. Hippocampal whole cell extracts (A) or synaptosome fractions (B) from wild-type and GSK3 knockin mice were prepared 24 h (A), or 30 min (B) after saline or ketamine (10 mg/kg) treatment and analyzed by multiplex for BDNF (A) and phospho-mTOR and mTOR (B). Means ± standard error (SEM). n = 3–6. (A) Two-way ANOVA (F1,24 interaction = 1.857, F1,24 treatment = 2.460, F1,24 genotype = 6.201); (B) two-way ANOVA (F1,15 interaction = 3.053, F1,15 treatment = 10.35, F1,15 genotype = 0.2794). *p < 0.05 compared to matched samples treated with saline.
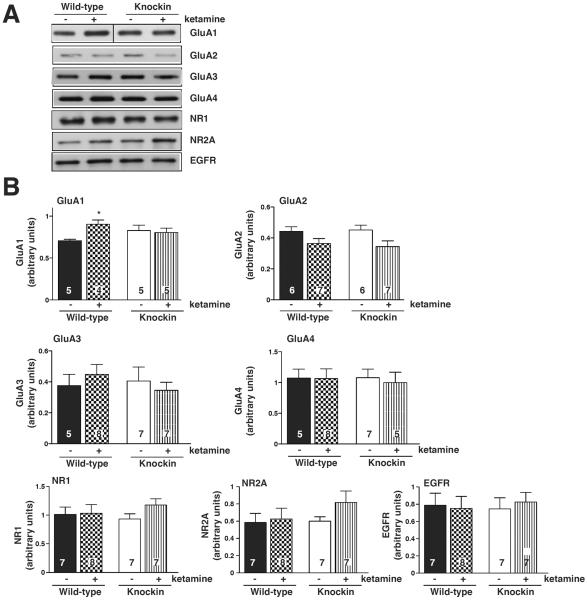
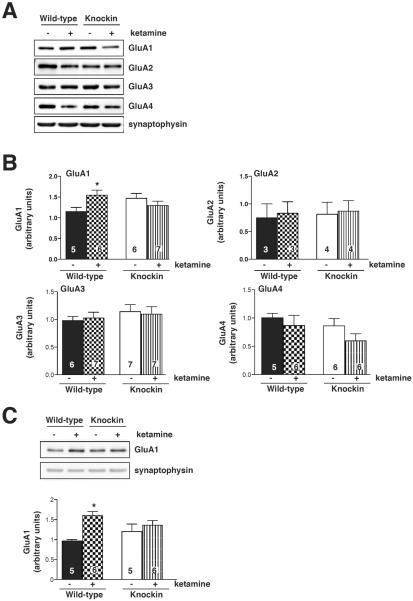
Because augmented signaling by AMPA receptors has been established to be required for the antidepressant response to ketamine, we next tested if ketamine-induced changes in the membrane trafficking of AMPA receptors were regulated by GSK3. Wild-type mice were treated with a sub-anesthetic, antidepressant dose of ketamine (10 mg/kg) and levels of AMPA receptor subunits were measured after 30 min in hippocampal membranes (Fig. 2) or hippocampal synaptosomes (Fig. 3). Ketamine treatment significantly increased the GluA1 subunit level, but did not alter hippocampal membrane or synaptosomal levels of GluA2, GluA3, or GluA4 (Fig. 2, Fig. 3, Supplementary Fig. S1). Since ketamine treatment causes inhibition of GSK3, and GSK3 promotes AMPA receptor internalization, we tested if the ketamine-induced increase in membrane GluA1 levels was dependent on inhibition of GSK3 by using GSK3 knockin mice in which ketamine cannot induce inhibitory serine-phosphorylation of GSK3. The ketamine-induced increase in hippocampal membrane and synaptosomal GluA1 levels was abrogated in GSK3 knockin mice, indicating that ketamine treatment increases GluA1 levels through inhibition of GSK3.
Fig. 2.
Ketamine-induced increased membrane glutamate receptor (GluA)1 localization in mouse hippocampus is blocked in glycogen synthase kinase-3 (GSK3) knockin mice. Hippocampal membranes from wild-type and GSK3 knockin mice were prepared 30 min after saline or ketamine treatment and immunoblotted for GluA1, GluA2, GluA3, and GluA4, NR1 and NR2A and for EGFR as loading control (A) and quantified in (B). Means ± standard error (SEM). n = 4–7. Two-way ANOVA (F1,15 interaction = 2.686, F1,15 treatment = 4.230, F1,15 genotype =2.109). *p < 0.05 compared to matched samples not treated with ketamine.
Fig. 3.
Ketamine-induced increased synaptosomal glutamate receptor (GluA)1 localization in mouse hippocampus is blocked in glycogen synthase kinase-3 (GSK3) knockin mice. Hippocampal synaptosomes from wild-type and GSK3 knockin mice were prepared 30 min after saline or ketamine treatment and immunoblotted for GluA1, GluA2, GluA3, and GluA4 and for synaptophysin as loading control (A) and quantified in (B). Means ± standard error (SEM). n = 3–7. Two-way ANOVA (F1,20 interaction = 6.374, F1,20 treatment = 0.9501, F1,20 genotype = 0.09833). *p < 0.05. Prefrontal cortex synaptosomes from wild-type and GSK3 knockin mice were prepared 30 min after saline or ketamine treatment and immunoblotted for GluA1 and for synaptophysin as loading control and quantified. Means ± SEM. n = 5–6. Two-way ANOVA (F1,18 interaction = 3.944, F1,18 treatment = 10.90, F1,18 genotype = 0.0016). *p < 0.05.
Hippocampal membrane levels of the NMDA receptor subunits NR1 and NR2A and the EGF receptor, used as a loading control, were also measured 30 min after administration of 10 mg/kg ketamine. None of these receptor levels were altered after ketamine treatment in either wild-type mice or GSK3 knockin mice, further demonstrating the selectivity of the response to ketamine on the GluA1 subtype of glutamatergic receptors (Fig. 2B).
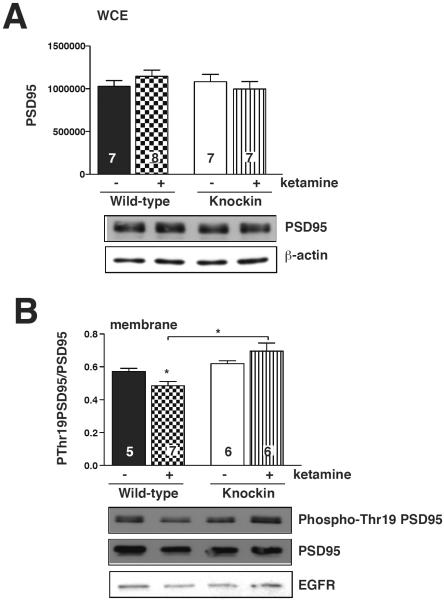
In order to determine the mechanism whereby inhibition of GSK3 promotes membrane accumulation of GluA1 in response to ketamine treatment, and since PSD-95 is phosphorylated by GSK3 and regulates AMPA receptor subunit trafficking, we tested if ketamine administration altered PSD-95 levels or phosphorylation in the hippocampus. The total level of PSD-95 in the hippocampus was unaltered by ketamine treatment, and was equivalent in wild-type and GSK3 knockin mice (Fig. 4A). However, ketamine treatment caused a reduction of PSD-95 phosphorylation on Thr-19 in hippocampal membranes, and this response to ketamine was blocked in GSK3 knockin mice, indicating the reduced membrane phospho-Thr19-PSD-95 was linked to ketamine-induced inhibition of GSK3 (Fig. 4B).
Fig. 4.
Phosphorylation of membrane post-synaptic density-95 (PSD-95) is reduced by ketamine treatment in mouse hippocampus. Wild-type and glycogen synthase kinase-3 (GSK3) knockin mice were treated with ketamine and hippocampi isolated. (A) Whole cell extracts (WCE) were immunoblotted for PSD-95. β-actin was used as loading control. (B) Hippocampal membrane preparations were immunoblotted for phospho-Thr19-PSD-95 and total PSD-95. EGFR was used as loading control. Means ± standard error (SEM). n = 5–7. Two-way ANOVA (F1,20 interaction = 6.354, F1,20 treatment = 0.1015, F1,20 genotype = 13.08). *p < 0.05.
Discussion
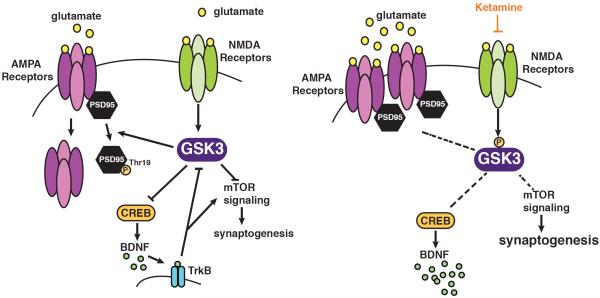
Substantial evidence indicates that the antidepressant action of ketamine involves augmentation of signaling through AMPA receptors (7). The findings reported here and previously support the model that GSK3 normally phosphorylates PSD-95 to promote AMPA GluA1 receptor subunit internalization, and that this is blocked by ketamine-induced inhibition of GSK3, resulting in increased cell surface localization of AMPA GluA1-containing receptors in the hippocampus (Fig. 5).
Fig. 5.
Scheme of the glycogen synthase kinase-3 (GSK3) signaling pathway modulated by ketamine. Prior to ketamine exposure, N-methyl-D-aspartate (NMDA) receptor activity maintains GSK3 active, which phosphorylates post-synaptic density-95 (PSD-95) to promote intracellular trafficking of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors. Ketamine blocks NMDA receptors, causing increased inhibitory serine-phosphorylation of GSK3, inhibiting its phosphorylation of PSD-95, leading to increased cellular membrane levels of the glutamate receptor (GluA)1 subunit of AMPA receptors. Dashed lines indicate signaling blocked by inhibition of GSK3 after ketamine treatment.
Ketamine treatment increases the inhibitory serine-phosphorylation of GSK3 (11, 28). Furthermore, previous studies have found that other NMDA antagonists, memantine (29), MK-801 (30, 31) and 7-chlorokynurenic acid (32), also increase the serine-phosphorylation of GSK3 and that NMDA treatment of cultured hippocampal neurons causes rapid dephosphorylation of phospho-Ser9-GSK3β (33, 34). Ketamine administration to drug-free depressed patients also rapidly increased the serine-phosphorylation of GSK3 (18). A recent report showed that a metabolite of ketamine, (2S,6S;2R,6R)-hydroxynorketamine, is a key mediator of the antidepressant actions of ketamine (35). Therefore it will be important to determine if this metabolite has an inhibitory effect on GSK3, and if it mediates the inhibition of GSK3 induced by ketamine. Adequate inhibition of GSK3 by ketamine appears to be an important component of the antidepressant response to ketamine for several reasons. Blockade of the ketamine-induced inhibition of GSK3 abrogates the antidepressant effect of ketamine in mouse models (11) and also blocked the ketamine-induced increases in hippocampal BDNF and phosphorylation of mTOR. Furthermore, co-administration of the GSK3 inhibitor lithium allows lower doses of ketamine to provide antidepressant effects in mouse models (19, 20). Also, administration of GSK3 inhibitors or molecular reduction of GSK3 is sufficient to induce rapid antidepressant effects in rodent models [reviewed in (15)].
The inhibition of GSK3 by ketamine was found to increase cell-surface localization of GluA1 AMPA receptors, which is important because increased AMPA receptor signaling has been demonstrated to be an important component of the antidepressant action of ketamine (8, 9, 22, 23). The results are consistent with the mechanism of ketamine-induced inhibition of GSK3 diminishing the internalization of AMPA GluA1 subunits, but other mechanisms may also contribute to the findings. For example, at later time points, the membrane expression of other AMPA receptors may also be modulated. Our findings indicate that one mechanism by which ketamine augments AMPA receptor-mediated signaling is by inhibiting the internalization of GluA1 AMPA receptors mediated by GSK3-induced Thr19 phosphorylation of PSD-95. Previous reports have shown that GSK3 promotes AMPA receptor trafficking (25, 36, 37). In particular, it was shown that GSK3 phosphorylates the postsynaptic protein PSD-95, which regulates AMPA receptor trafficking (27). Thus, the present results indicate that ketamine-induced inhibition of GSK3 reduces the Thr19 phosphorylation of PSD-95, which normally promotes internalization of AMPA receptors, augmenting cell surface localization of GluA1 AMPA receptors following ketamine administration (Fig. 5). We focused on the hippocampus since the hippocampus has been implicated on mediating many depression-like behaviors, and that the action of GSK3 in regulating PSD-95 was reported in the hippocampus (27). These findings are also in accordance with studies of the regulatory role of GSK3 on synaptic plasticity mediated by long-term depression (LTD). The transient activation of NMDA receptors during LTD leads to the internalization of AMPA receptors from the cell surface, a process that is dependent on active GSK3 (38). Thus, GSK3 inhibitors block LTD, and it was recently found that a low concentration of ketamine, relevant for its antidepressant actions, blocks LTD (39). Thus, ketamine's antidepressant effects and inhibition of LTD appear to share the common synaptic plasticity regulatory pathway of GSK3 inhibition and augmentation of signaling by AMPA receptors on the cell surface. This also raises the possibility that diminished hippocampal LTD is directly involved in the contributing to the antidepressant action of ketamine, in accordance with converse evidence that hippocampal LTD is enhanced by a variety of stresses that induce depression-like behaviors of rodents (40–43).
Conclusions
Altogether, these findings show that administration of an antidepressant dose of ketamine increases cell membrane GluA1 levels by inhibiting GSK3, demonstrating a mechanistic contribution to the role of GSK3 inhibition in the rapid antidepressant action of ketamine.
Supplementary Material
Acknowledgements
This research was supported by grants from the National Institute of Mental Health (MH038752, MH090236, MH095380, MH104656) and a Young Investigator Award from the Brain & Behavior Research Foundation.
Footnotes
Disclosures The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 3.Fond G, Loundou A, Rabu C, Macgregor A, Lancon C, Brittner M, et al. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology. 2014;231:3663–3676. doi: 10.1007/s00213-014-3664-5. [DOI] [PubMed] [Google Scholar]
- 4.Martinowich K, Jimenez DV, Zarate CA, Jr., Manji HK. Rapid antidepressant effects: moving right along. Molecular psychiatry. 2013;18:856–863. doi: 10.1038/mp.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niciu MJ, Henter ID, Luckenbaugh DA, Zarate CA, Jr., Charney DS. Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds. Annual review of pharmacology and toxicology. 2014;54:119–139. doi: 10.1146/annurev-pharmtox-011613-135950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdallah CG, Averill LA, Krystal JH. Ketamine as a promising prototype for a new generation of rapid-acting antidepressants. Ann N Y Acad Sci. 2015;1344:66–77. doi: 10.1111/nyas.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zunszain PA, Horowitz MA, Cattaneo A, Lupi MM, Pariante CM. Ketamine: synaptogenesis, immunomodulation and glycogen synthase kinase-3 as underlying mechanisms of its antidepressant properties. Molecular psychiatry. 2013;18:1236–1241. doi: 10.1038/mp.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biological psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16:1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacology & therapeutics. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, et al. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. The EMBO journal. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polter A, Beurel E, Yang S, Garner R, Song L, Miller CA, et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35:1761–1774. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jope RS. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Frontiers in molecular neuroscience. 2011;4:16. doi: 10.3389/fnmol.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang C, Zhou ZQ, Gao ZQ, Shi JY, Yang JJ. Acute increases in plasma mammalian target of rapamycin, glycogen synthase kinase-3beta, and eukaryotic elongation factor 2 phosphorylation after ketamine treatment in three depressed patients. Biological psychiatry. 2013;73:e35–36. doi: 10.1016/j.biopsych.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK. GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology. 2013;38:2268–2277. doi: 10.1038/npp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu CT, Scheuing L, Liu G, Liao HM, Linares GR, Lin D, et al. The mood stabilizer lithium potentiates the antidepressant-like effects and ameliorates oxidative stress induced by acute ketamine in a mouse model of stress. Int J Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annual review of neuroscience. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 22.Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behavioural brain research. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 23.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Du J, Gray NA, Falke CA, Chen W, Yuan P, Szabo ST, et al. Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6578–6589. doi: 10.1523/JNEUROSCI.1258-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du J, Wei Y, Liu L, Wang Y, Khairova R, Blumenthal R, et al. A kinesin signaling complex mediates the ability of GSK-3beta to affect mood-associated behaviors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11573–11578. doi: 10.1073/pnas.0913138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gould TD, O'Donnell KC, Dow ER, Du J, Chen G, Manji HK. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology. 2008;54:577–587. doi: 10.1016/j.neuropharm.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson CD, Kim MJ, Hsin H, Chen Y, Sheng M. Phosphorylation of threonine-19 of PSD-95 by GSK-3beta is required for PSD-95 mobilization and long-term depression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:12122–12135. doi: 10.1523/JNEUROSCI.0131-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou W, Dong L, Wang N, Shi JY, Yang JJ, Zuo ZY, et al. Akt mediates GSK-3beta phosphorylation in the rat prefrontal cortex during the process of ketamine exerting rapid antidepressant actions. Neuroimmunomodulation. 2014;21:183–188. doi: 10.1159/000356517. [DOI] [PubMed] [Google Scholar]
- 29.De Sarno P, Bijur GN, Zmijewska AA, Li X, Jope RS. In vivo regulation of GSK3 phosphorylation by cholinergic and NMDA receptors. Neurobiology of aging. 2006;27:413–422. doi: 10.1016/j.neurobiolaging.2005.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo MS, Kim SH, Ahn YM, Kim Y, Jeon WJ, Yoon SC, et al. The effects of repeated administrations of MK-801 on ERK and GSK-3beta signalling pathways in the rat frontal cortex. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2007;10:359–368. doi: 10.1017/S1461145706006869. [DOI] [PubMed] [Google Scholar]
- 31.Yu J, Qi D, Xing M, Li R, Jiang K, Peng Y, et al. MK-801 induces schizophrenic behaviors through downregulating Wnt signaling pathways in male mice. Brain research. 2011;1385:281–292. doi: 10.1016/j.brainres.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 32.Zhu WL, Wang SJ, Liu MM, Shi HS, Zhang RX, Liu JF, et al. Glycine site N-methyl-D-aspartate receptor antagonist 7-CTKA produces rapid antidepressant-like effects in male rats. Journal of psychiatry & neuroscience : JPN. 2013;38:306–316. doi: 10.1503/jpn.120228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo HR, Hattori H, Hossain MA, Hester L, Huang Y, Lee-Kwon W, et al. Akt as a mediator of cell death. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11712–11717. doi: 10.1073/pnas.1634990100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng WH, Quirion R. Glutamate acting on N-methyl-D-aspartate receptors attenuates insulin-like growth factor-1 receptor tyrosine phosphorylation and its survival signaling properties in rat hippocampal neurons. The Journal of biological chemistry. 2009;284:855–861. doi: 10.1074/jbc.M807914200. [DOI] [PubMed] [Google Scholar]
- 35.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang MJ, Li YC, Snyder MA, Wang H, Li F, Gao WJ. Group II metabotropic glutamate receptor agonist LY379268 regulates AMPA receptor trafficking in prefrontal cortical neurons. PloS one. 2013;8:e61787. doi: 10.1371/journal.pone.0061787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei J, Liu W, Yan Z. Regulation of AMPA receptor trafficking and function by glycogen synthase kinase 3. The Journal of biological chemistry. 2010;285:26369–26376. doi: 10.1074/jbc.M110.121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, et al. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 39.Izumi Y, Zorumski CF. Metaplastic effects of subanesthetic ketamine on CA1 hippocampal function. Neuropharmacology. 2014;86:273–281. doi: 10.1016/j.neuropharm.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaouloff F, Hemar A, Manzoni O. Acute stress facilitates hippocampal CA1 metabotropic glutamate receptor-dependent long-term depression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:7130–7135. doi: 10.1523/JNEUROSCI.1150-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holderbach R, Clark K, Moreau JL, Bischofberger J, Normann C. Enhanced long-term synaptic depression in an animal model of depression. Biological psychiatry. 2007;62:92–100. doi: 10.1016/j.biopsych.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Marsden WN. Synaptic plasticity in depression: molecular, cellular and functional correlates. Progress in neuro-psychopharmacology & biological psychiatry. 2013;43:168–184. doi: 10.1016/j.pnpbp.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Pignatelli M, Vollmayr B, Richter SH, Middei S, Matrisciano F, Molinaro G, et al. Enhanced mGlu5-receptor dependent long-term depression at the Schaffer collateral-CA1 synapse of congenitally learned helpless rats. Neuropharmacology. 2013;66:339–347. doi: 10.1016/j.neuropharm.2012.05.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.