Fig. 5.
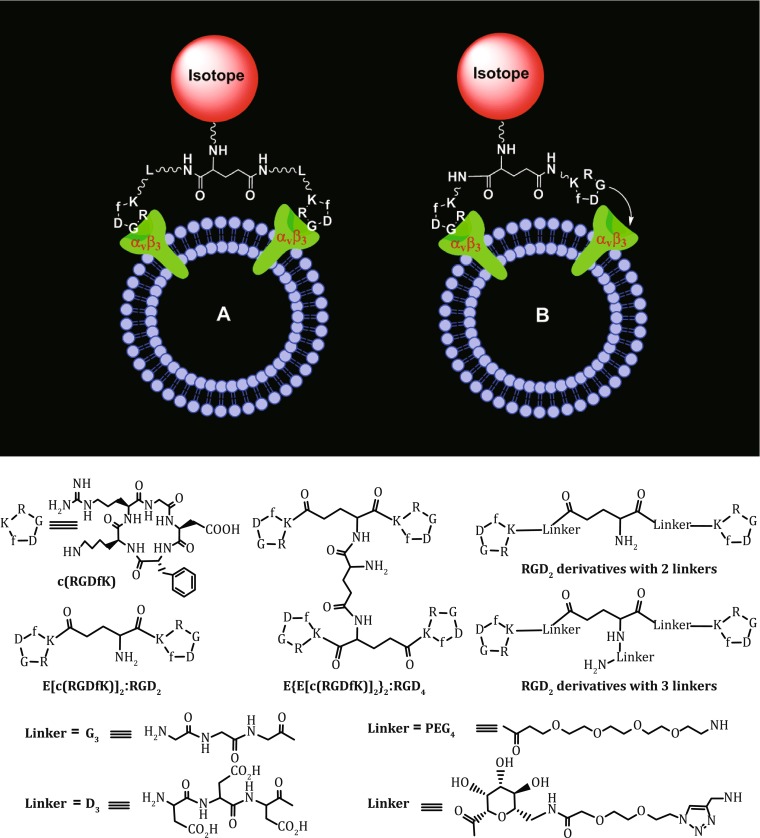
Top: Schematic illustration of the interactions between cyclic RGD peptide dimers and αvβ3. A The distance between two RGD motifs is not long enough for simultaneous integrin αvβ3 binding. However, the RGD concentration is “locally enriched” in the vicinity of neighboring integrin αvβ3 once the first RGD motif is bound. B The distance between two RGD motifs is long due to the presence of two linkers (L). As a result, the cyclic RGD dimer is able to bind integrin αvβ3 in a “bivalent” fashion. In both cases, the end-result would be higher integrin αvβ3 binding affinity for the multimeric cyclic RGD peptides. Bottom: Selected cyclic RGD peptide dimers and tetramers useful for development of αvβ3-targeted radiotracers. The D3, G3, PEG4, and sugar linkers are used to increase the distance between two RGD motifs and to improve radiotracer excretion kinetics from non-cancerous organs
