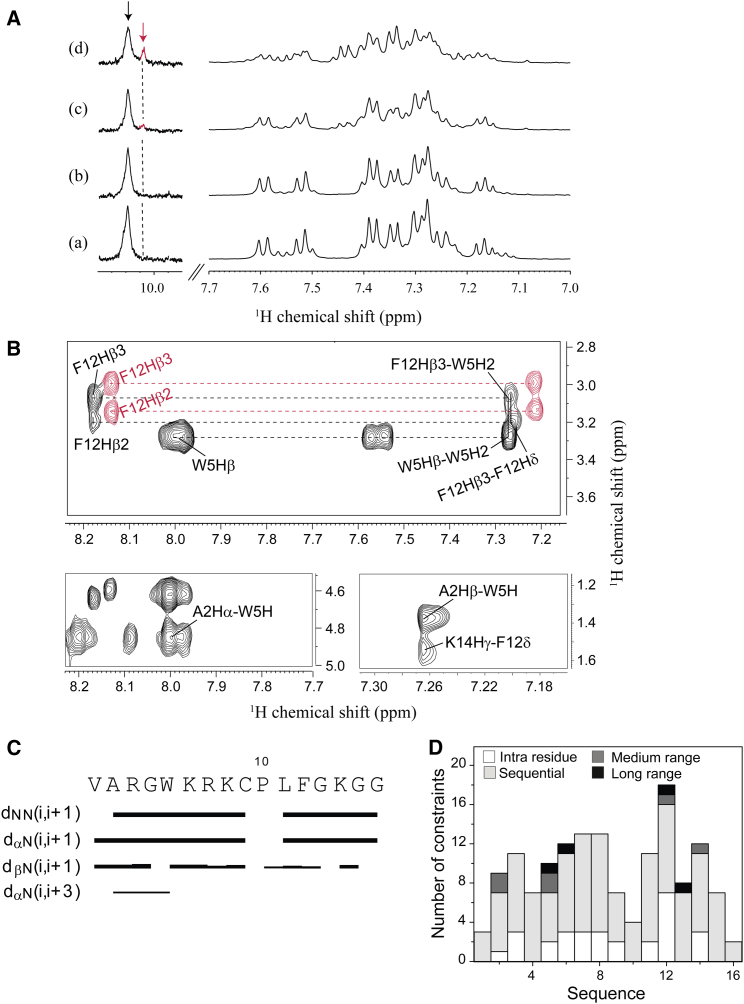
Figure 6.
1D and 2D NMR analysis of VG16KRKP interaction with C. neoformans and its structure calculation. (A) 1D proton NMR spectra of VG16KRKP alone (a) and upon titration with increasing concentrations of C. neoformans cells (b–d) showing line broadening of the proton resonances and reduction in intensity indicating binding along with the emergence of a small peak in the Trp indole region (d) indicating the presence of another conformation. (B) Different regions of the tr-NOESY spectra showing the important medium- and long-range NOE connectivities along with presence of two different conformations (red labels indicate conformation 1 and black labels indicate conformation 2). The experiment was performed at 25°C and pH 5.5 using a 700 MHz Bruker Avance III NMR spectrometer, equipped with a cryoprobe. (C) Bar plot showing NOE connectivities of the bound conformation 2 of VG16KRKP with the thickness of the lines corresponding to the number of NOEs; (D) histogram showing the short-, medium-, and long-range NOEs of VG16KRKP conformation 2 when bound to C. neoformans. To see this figure in color, go online.