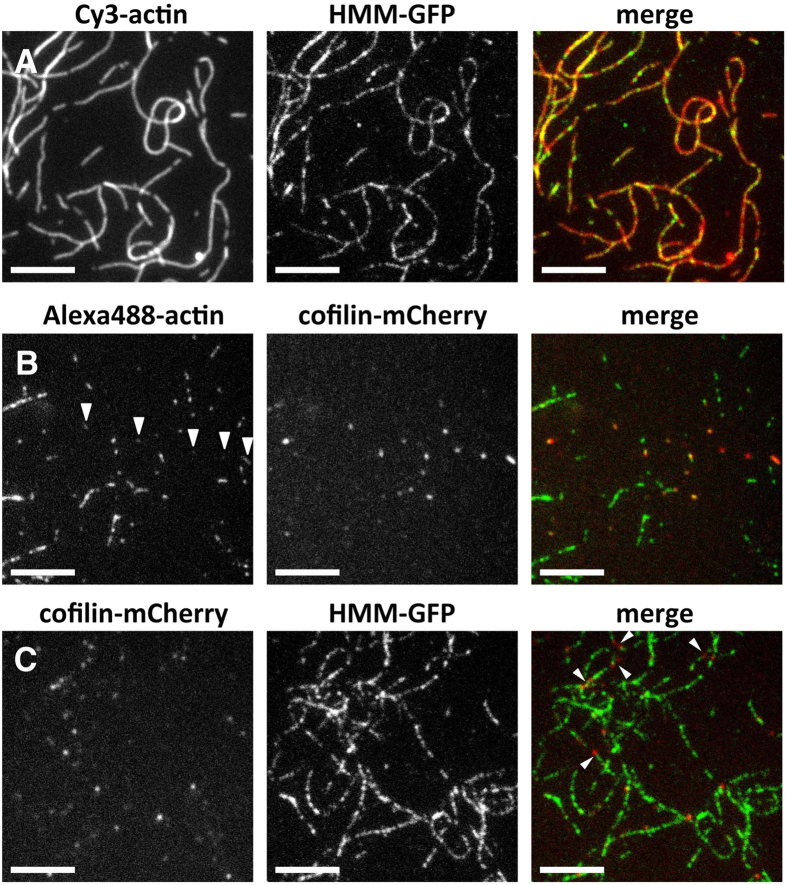
Figure 1. Fluorescence microscopic observation of binding of Dd HMM-GFP and Hs cofilin-mCherry to Dd actin filaments.
In all experiments, actin filaments were loosely immobilized on a positively-charged lipid bilayer and the binding reaction was performed in F-buffer 1 containing 0.2 μM ATP. (A) Cooperative binding of 7 nM HMM-GFP along Cy3-labeled actin filaments, forming short clusters or puncta along the actin filaments. (B) Cooperative binding of 80 nM cofilin-mCherry to Alexa488-labeled actin filaments. Cofilin-mCherry formed tight clusters along filaments. Alexa488 fluorescence of filaments bound with cofilin-mCherry was weaker (arrowheads), presumably due to quenching by FRET with mCherry. (C) Simultaneous addition of 7 nM HMM-GFP and 80 nM cofilin-mCherry to unlabeled actin filaments. Because the concentrations of actin filaments and HMM-GFP were identical to those in (A), most of the filaments were labeled by HMM-GFP in a punctate manner. Other shorter filaments were labeled only by cofilin-mCherry. Additionally, some filaments were labeled by both fluorescent proteins in an alternating punctate manner (arrowheads). Scale bars: 5 μm.