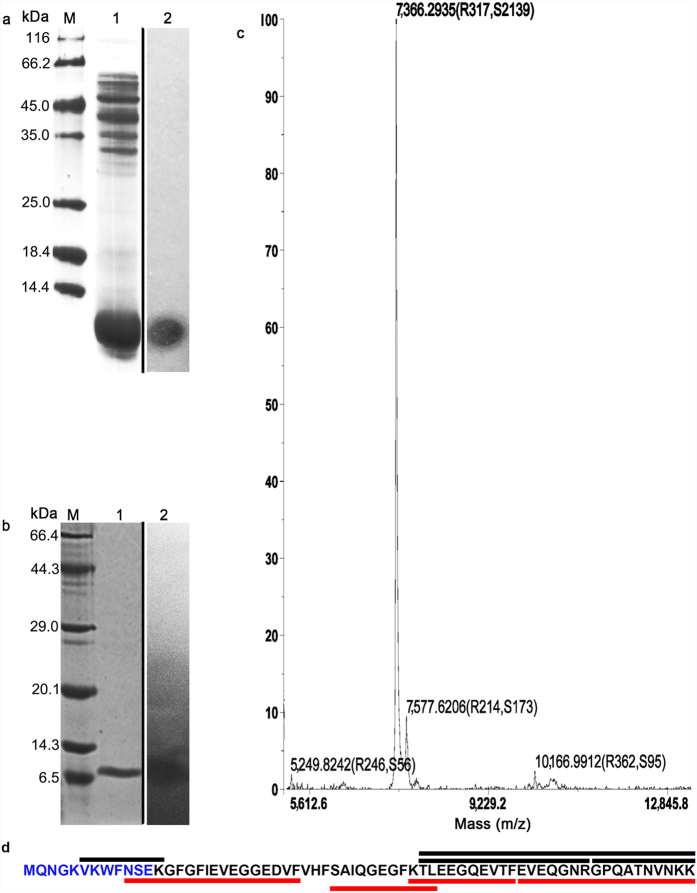
Figure 2. Determination of the primary structure of BtCspB.
(a) The tricine-SDS-PAGE of Bt BRC-ZYR2 bacteriocins and the detection of their antibacterial activity in situ on a gel. Lane M: Fermentas unstained protein molecular weight marker (SM0431). Lane 1: Bt BRC-ZYR2 bacteriocins precipitated by 100% ammonium sulfate. Lane 2: Antibacterial activity of Bt BRC-ZYR2 bacteriocins detected in situ on gel. The samples had been run in a same tricine-SDS-PAGE gel under the same experimental conditions. After electrophoresis, the gel was cut vertically. The first part containing a protein marker (Lane M) and the purified bacteriocins (Lane 1) were stained with Coomassie brilliant blue (CBB) R-250 to estimate the MW. The second part containing the purified bacteriocins (Lane 2) was assayed for the direct detection of bacteriocin activity by the presence of an inhibition zone. The borders of two parts were clearly demarcated in the figure by setting up a black line. (b) The protein profile of BtCspB purified with a second round of gel filtration using cellulose DEAE-52 anion-exchange chromatography. Lane M: TaKaRa premixed protein marker (Broad). Lane 1: BtCspB purified with a second round of gel filtration using cellulose DEAE-52 anion-exchange chromatography. Lane 2: Antibacterial activity of BtCspB detected in situ on a gel. The samples had been run as (a). (c) The molecular mass determination of BtCspB by AB SCIEX 5800 MALDI-TOF/TOF MS. A major peak at 7366.2935 Da represents the molecular ion peak. (d) The coverage map of BtCspB by N-terminal amino acid sequencing and LC-MS/MS. The blue characters were the 12 N-terminal amino acids sequenced by Edman degradation (see Supplementary Fig. 4). The characters with a black overline corresponded to the tryptic peptides identified by LC-MS/MS (see Supplementary Fig. 5a), whereas the characters with a red underline represented the chymotryptic peptides identified by LC-MS/MS (see Supplementary Fig. 5b).