Figure 1.
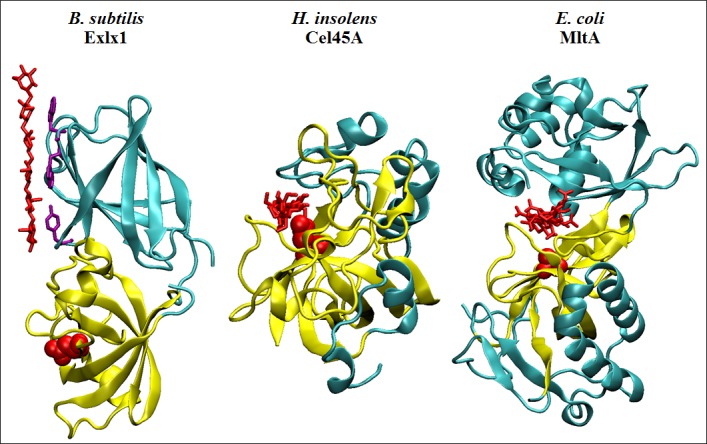
Crystal structures of proteins sharing a six‐stranded double psi beta barrel fold (yellow) interacting with oligosaccharide molecules (red). Bacillus subtilis Exlx1 (PDB: 4FER) shows a glycan chain interacting through its aromatic residues (purple) on the surface of D2. Humicola insolens Cel45A (PDB: 4ENG) forms an active site residing along the enzyme's surface where a cellohexaose molecule (viewed from the side) is stabilized by hydrogen bonds with residues forming the catalytic cleft. Escherichia coli MltA (PDB: 2PI8) shows an enzyme monomer complexed with a chitohexaose molecule (also viewed from the side) that interacts at the interface between its two domains, which is tightly bound in a deep and narrow active site groove. Active aspartates D82 and D121 (from BsExlx1 and HiCel45A, respectively), and the position of the active D308 of EcMltA (mutated by an alanine in this structure) are highlighted in red.
