Table 2.
Optimizations of catalyst and reaction conditions for enantioselective isomerization of 1aa
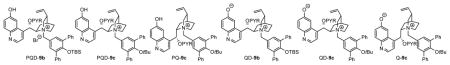
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | cat. | mol% of cat. | Base presentedin the reaction | Solvent | t (h) | conv. (%)b | ee (%)b |
| 1 | QD-9a | 0.5 | No | Toluene/CHCl3 = 7/3 | 4 | 76 | 92 |
| 2 | QD-9b | 0.5 | No | Toluene/CHCl3 = 7/3 | 4 | 97 | 93 |
| 3 | QD-9c | 0.5 | No | Toluene/CHCl3 = 7/3 | 4 | 98 | 95 |
| 4 | QD-9c | 0.2 | No | Toluene/CHCl3 = 7/3 | 24 | 77 | 95 |
| 5 | OD-9c | 0.2 | solid K2CO3 | Toluene/CHCl3 = 7/3 | 12 | 100 | 95 |
| 6 | QD-9c | 0.02 | solid K2CO3 | Toluene | 24 | 100 | 96c |
| 7 | QD-9c | 0.01 | solid K2CO3 | Toluene | 24 | 97 | 96 |
| 8 | Q-9c | 0.05 | solid K2CO3 | Toluene | 24 | 100 | −93 |
Betaine catalysts QD-9 and Q-9c were preformed from treatment of the corresponding precursors PQD-9 and PQ-9c with base (see supporting information for details). Reactions were run with 1a (0.025 mmol) in solvent (0.25 mL) indicated. The ratio of 2a/4a was determined to be greater than 99/1 by HPLC analysis.
Determined by HPLC analysis.
Absolute configuration was determined to be R. See Supporting Information for details.
