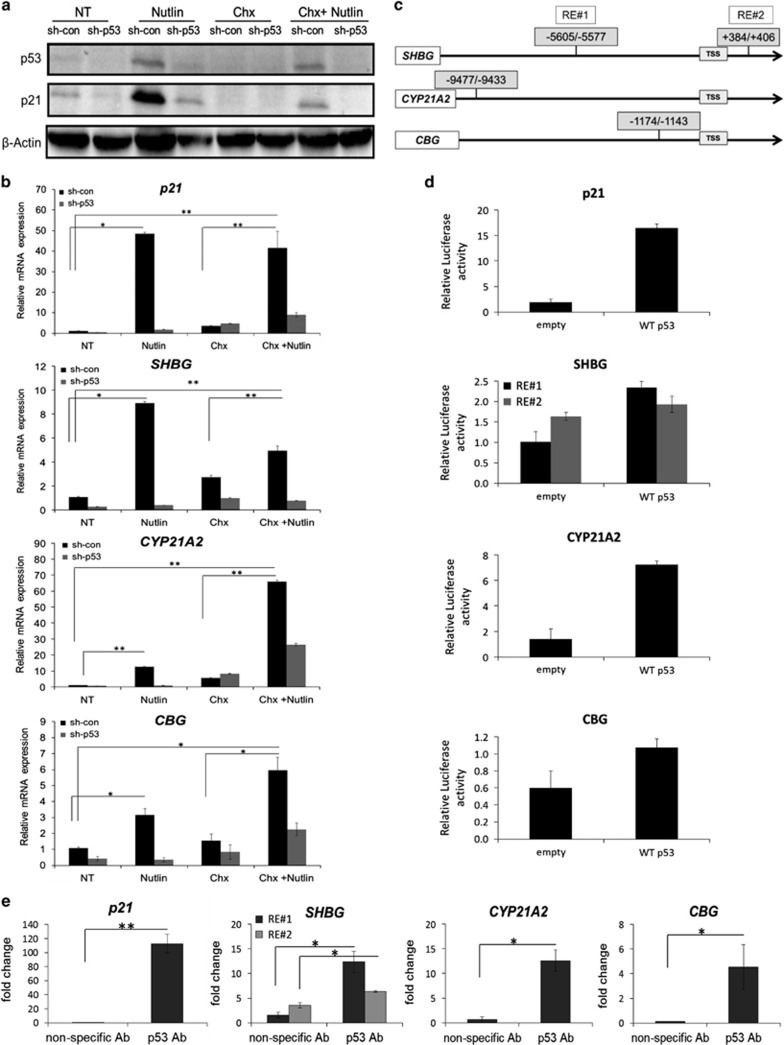
Figure 4.
p53 directly induces SHBG, CYP21A2 and CBG transcription by binding to specific p53 REs entailed in their loci. HepG2 sh-con/sh-p53 cells were maintained in CON medium, NT or treated with either 10 μM Nutlin-3a for 24 h (Nutlin) or with 10 μg/ml Chx for 6 h (Chx) alone or with a combination of Nutlin-3a for 18 h followed by Chx treatment for additional 6 h (Chx+Nutlin). (a) Protein levels of p53 and p21 were measured by western blot. β-Actin was used as loading CON. (b) qRT-PCR analysis of p21, SHBG, CYP21A2 and CBG genes. (c) SHBG, CYP21A2 and CBG genomic sequences were analyzed by p53MH algorithm. Representative scheme of the potential p53 REs locations relative to TSS are indicated. (d) Reporter assay analysis of p53 REs in SHBG, CYP21A2 and CBG promoters. H1299 cells were transiently co-transfected with Luciferase reporter construct harboring distinct p53 REs of the steroid hormone binding factors loci as well as with WT p53 expression vector or corresponding empty vector as CON. Luminescence values were estimated 48 h after transfection. Transfection efficacy was normalized on the basis of the activity of the co-transfected Gaussia luciferase. (e) ChIP analysis of HepG2 cells treated with 10 μM Nutlin-3a for 24 h. p53 protein was immunoprecipitated using p53-specific H47 polyclonal antibody (p53 Ab). Nonspecific antibody-IgG was used as a CON (nonspecific Ab). qRT-PCR was performed using specific primers against the p53 REs indicated in (c). Values were normalized to 1% input of the corresponding sample. Results are of a representative experiment from at least three experiments. *P<0.05, **P<0.01, determined by Student's t-test analysis