Abstract
Background
Most spinal cord infarctions are due to aortic pathologies and aortic surgeries. Fibrocartilaginous Embolism (FCE) has been reported to represent 5.5% of spinal cord infarctions. Some believe that FCE is more common than presumed and is rather under-diagnosed due to vagueness surrounding its clinical presentation.
Method
A literature search was conducted for case reports of FCE published before August 2014. PubMed, the Cochrane Central Register and Google Scholar were searched for different combinations of the key words "fibrocartilaginous, "nucleus pulposus", "embolism", "spinal cord", "inter-vertebral disc", "infarction", "stroke", "paraplegia", "quadriplegia", "myelopathy".
Result
Fifty-five case articles were reviewed, ten of which were translated from foreign languages. A total of 67 cases of FCE were found, 41 tissue-confirmed and 26 clinically suspected. A comprehensive summary of the clinical anatomy, patho-physiologic mechanisms, epidemiology, diagnosis and treatment of FCE is described, along with the conflicting opinions on its incidence and relevance after reviewing all of the related literature. The 41 tissue proven cases are summarized and a schematic approach to the clinical diagnosis of FCE, deducted from their clinical findings, is presented.
Conclusion
FCE of the spinal cord, often mis-diagnosed as transverse myelitis, may be more common than presumed. Future research into FCE, including the development of a chondrolytic therapy that can be given empirically upon its clinical suspicion to acutely reverse its symptoms, may be of value.
Keywords: Nucleus pulposus, Paraplegia, Transverse myelitis, Stroke, Idiopathic myelopathy
Introduction
Fibrocartilaginous Embolism (FCE) refers to the migration of fibrocartilaginous nucleus pulposus material through the nearby vasculature to embolize into one of the spinal cord vessels. It was first described by Naiman1 in 1961 in a 15-year-old boy who developed quadriplegia shortly after suffering a trivial fall on his back during a basketball game. Since then several cases have been reported. FCE has been shown to cause embolic infarction most commonly to the spinal cord, but also to the lung,2 brain,3 vertebrae and ribs.4 FCE is also well described in the veterinary literature, most commonly occurring in dogs, and sporadically in other species.5 FCE to the spinal cord in humans has been reported in 41 histo-pathologically confirmed (Table 1) and 26 clinically suspected3 cases.
Table 1.
Histo-pathologically confirmed cases of FCE of the spinal cord
| Case-Author- Year | Age- Sex | PMH | Suspected Trigger | Time From Trigger to Onset/Time to Symptom Peak/Time to death | Pain-Radiation | Paralysis | CSF | Level of Infarction | Arterial vs Venous (Territory) | Degenerative Disc Ds / Level | Schmorl's Node |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-Naiman-19611 | 15-M | _ | Falling on buttocks | 20 m/1 hr/3 hr | Neck, back, shoulder | Quadriplegia | N | MO-C2-T7 | A(ASA) | No | _ |
| 2-Laterre-19628 | 31-F | None | None9 | -/2 hr/3 mo | Mid Back | Paraplegia | N | C6-T1 | A(ASA) | No | No |
| 3-Feigin-196510 | 49-F | C discectomy 2 yrs prior | _ | - /3 wk/6 wk | Left shoulder | Paraplegia, UE Monoparesis | N | C6-T1 | V(ASA) | Yes/C | Yes |
| 4-Feigin-196510 | 39-F | None, Diarrhea 2 days prior | _ | -/30 m/ 4 mo | Left shoulder | Paraplegia, UE Monoparesis | N | C4-T2 | A-V(PSA) | Yes/- | Yes |
| *5- Feigin-196510 | 55-M | Portal Cirrhosis | Portal Hypertension | _ | None* | None* | _ | Sacral* | V* | _ | _ |
| 6-Bodechtel- 196811 | 28-F | Pregnancy,7th mo | _ | -/ 12 hr/ 6 d | _ | R Hemiplegia | N | MO-Conus | Venous ** | Yes/- | No |
| 7-Bruno-196912 | 47-F | Htn, Obesity | _ | -/24 hr/ 142 d | Lt chest, neck, mid back | Paraparesis,UEMonoparesis | N | C1–7 | ASA(A and V) | Yes/T6 (& T6 Fracture) | - |
| 8- Lvovskiy- 196913 | 21-M | ? | Extended Neck | ?/ 4 hr/ 5 d | Neck | ? | ? | C2-C7 | A(ASA) | ? | ? |
| 9-Jurkovic-197014 | 66-F | None | Shopping &heavy lifting with 2arms | 1 d /few days/ 18 d | Lower Back to LE's | Paraparesis | _ | C7, T4, T8, T10, L2 | V(ASA) | _ | Yes/L (4 nodes) |
| 10-Kepes-197315 | 38-F | None | MVA 3 wks prior | 3 wk/1 hr/ 40 d | R posterior neck | Quadriplegia | N | MO-C4 | A-V(ASA) | No | Yes |
| 11-Hubert-197416 | 63-F | Obesity | Episode of coughing17 | minutes/?/ Some Hrs | Neck | Quadriparesis except R LE. | N | C3-C7 | A-V(ASA) | No | No |
| 12- Hubert- 197418 | 17-F | _ | Years of riding | -/ 48 hr/ 11 d | Neck | Quadriplegia | N | C4-T6 | A(ASA) | No | No |
| 13-Roitzsch-197519 | 69-F | _ | Fall on back17 | Minutes/- / 11 mo | _ | Paraplegia | N | T12-L4 | A-V(ASA) | No | _ |
| 14- Peiffer-197620 | 36-M | _ | Fall forward17 | -/ 48 hr/ 2 mo | LowerBack,R hip | Paraplegia | N | ? | A-V ** | Yes/C5–6 | No |
| 15-Hanski-197721 | 51-M | Smoker, PAD | Loading Goods17 | -/ -/ 4 d | Chest pain | Paresis of UE's | ↑Ptn (47) | C5-T1 | A(ASA) | No | No |
| 16- Schairer-197722 | 19-F | Obesity | _17 | -/ 3 hr/ 9 mo | No mentioning | Quadriplegia. | N | C4-T-L | A(ASA) | _ | _ |
| 17- Budka- 197923 | 49-F | LBP24 | _17 | -/ 10 hr/ 14 wk | Lower back | Paraparesis. | N | T11-Conus | A-V(PSA) | No | No |
| 18-Ho-19809 | 22-F | None | Stooping | 15 m/30 m/9 d | Back of neck | Paraplegia | N | C3-T1 | A(ASA) | No | No |
| 19-Bots-198117 | 29-F | _ | None | -/2–5 hr/ 15 d | Back | Quadriplegia | N | MO,C1-T5 | A | No | _ |
| 20- Bots- 198117 | 32-F | _ | _ | /3 hr/ 11 mo | Shoulders | Quadriplegia | N | MO, C1-T5 | A(ASA) | No | _ |
| 21- Bots- 198117 | 77-M | DM | _ | -/ hrs/ 22 hr | Back of neck | Quadriplegia | N | C1-C4 | A(ASA) | No | _ |
| 22- Bots- 198117 | 60-F | _ | None | -/ -/3 mo | _ | Paraplegia | _ | T10-T11 | A(ASA) | _ | _ |
| 23- Srigley- 198125 | 46-M | DM,Hypothyroid, Sarcoid | Extended neck during shaving | Minutes/ 17 hr/ 1 mo | Neck | Quadriplegia | ↑Ptn (45) | C1-C4 | A(ASA) | Yes/C | _ |
| 24-Kase-198326 | 23-F | _ | None | -/minutes/ 12 d | Occipital HA | Quadriplegia | N | MO-C5 | A(ASA) | No | _ |
| 25-Barz-198627 | 56-F | _ | Jump from 80 cm window | 15–30 m/2 hr/ 13 d | _ | Paraplegia | ↑Ptn (50) | T12 -Conus | A(ASA) | Yes | Yes/T12 |
| 26-Barz-198928 | 77-F | Yrs of LBP | Shopping exercise/Strain | 4 hr/8 hr/14 d | Back then R leg | Paraperesis | N | S-Conus | A(ASA) | _ | No |
| 27-Banerjee-198929 | 21-M | None | None | -/2 hr/2 d | Neck to Lt arm | Quadriplegia | _ | C2-C6 | A(ASA) | No | - |
| 28- Kestle-198930 | 43-M | LBP × 3 wk | _ | -/2 d/ 2 wk | Butocks& thigh | Paraparesis | ↑Ptn (50) | T12-Conus | A(ASA,PSA) | Yes/T9-L1 | Yes/T9-L1 |
| 29-Bockenek-199031 | 20-M | _ | MVA & hearing a snap in his spine | 24 hr/1 hr/6.5 yr | Neck to 4 limbs | Quadriplegia | _ | C4-T4 | Vessels | No | _ |
| 30-Scully -199132 | 13-M | MVA × 1 yr | Somersaults | 10–20 m/ 6 hr/- | Neck | Hemiparesis. | _ | C2–4 | A(ASA) | No | _ |
| 31-Scully -199132 | 61-F | Htn × 10 yrs | Grocery Shopping | 1–2 hr/3 hr/- | No mentioning | Paraplegia | N | T11- L1 | A-V(ASA) | _ | _ |
| 32-Moorhouse-199233 | 63-F | _ | Strain with defecation | 10–20 m/30 hr/hrs | Mid& Lower Back | Paraplegia | N | T11-L1 | A(ASA) | Yes/L4–5 | No |
| 33-Mikulis- 199234 | 61-F | Htn, Obesity, LBP × 4 mo 24 | _ | _ | Lower Back | Paraplegia | N (only ↑RBC 68/mm3) | T11-L1 | A-V(ASA) | Yes | No |
| 34-Toro -199424 | 16-F | None | Stooping to milk a cow | 1–10 m/15 m/6 wk | LBP to thighs | Paraplegia | ↑Ptn | L1 | A-V | No | No |
| 35-Yousef -199835 | 14-F | Obese (86 kg, 1.6 m, 33.6 BMI) | Leaning forward to pick up an object | Immediate/<24 hr/ 5 d | Mid back | Paraplegia | N | T5–7 | A(ASA) | No | Yes |
| 36-Freyaldenhoven -200136 | 19-M | None | Hit on the back by a large door | 2 d/-/- | Back | Quadriplegia | N | C | A | No | _ |
| 37-Alexander-200337 | 60-F | Chronic LBP | Swimming | minutes/- /17 d | Funny lower back sensation | Paraplegia | N | T9–11 | A(ASA) | Yes/L3-L5 | Yes/L4 |
| 38- Uppal - 200438 | 41-F | Pregnancy, 25th wk | _ | -/<30 m/22 d | _ | Paraplegia | N | T6–7 to Conus | A(PSA) | Yes/C3–5 | Yes |
| 39- Duprez- 200539 | 78-M | Htn | Minor fall | Few days/6 hr/28 d | _ | Paraplegia. | ↑Ptn (58) | Conus | A | Yes | Yes |
| 40-Meyer-200540 | 66-M | _ | Paravertebral C5–6 injection | 2.5 hr/2 d/61 d | _ | Quadriparesis | _ | C2–3 | A(ASA) | No | _ |
| 41-Piao- 200941 | 23-M | _ | Trivial strike to neck and back | 10 d/-/3 mo | Mid Back | Quadriplegia. | Cells 20/mm3, MBP 22 nmol/l | MO - C7 | A-V(ASA) | Yes/C7-T1 | _ |
A=arterial; ASA=anterior spinal artery; C=cervical; d=days; DM=diabetes mellitus; Ds=disease; F=female; FCE=fibrocartilaginous embolism; HA=headache; Htn=hypertension; hr=hours; L=lumbar; LBP=lower back pain; LE=lower extremity; Lt=left; M=male; m=minutes; mo=months; MO=medulla oblangata; MVA=motor vehicle accident; N=normal; PAD=peripheral arterial disease; PSA=posterior spinal artery; Ptn=protein (mmol/L); R=right; S=sacral; T=thoracic; UE=upper extremity; V=venous; wk=weeks; " -- "= no report in the literature to confirm or deny, "?"= unclear translation.
Clinical Anatomy
Despite being referred to as “the largest avascular structure in the body,” the inter-vertebral disc can indeed be the source of embolic material as evidenced by histo-pathologic sectioning and staining in all cases mentioned in Table 1. The inter-vertebral disc is classically divided into an outer mesodermally derived annulus fibrosus, and a central endodermally derived nucleus pulposus.6 In neonates, the inter-vertebral disc is a highly vascular structure with large thin walled blood channels running mainly in the cartilage end plate.6,7 This vascular tissue quickly starts to regress after 2 months and throughout the first decade of life. Eventually, by age 11–16 years will have completely disappeared.7
Neo-vascularization reappears in the normal adult inter-vertebral disc at the circumferential edges at around 50 years of age.7 In individuals with degenerative disc disease, this revascularization has been demonstrated to occur much earlier and is more pronounced.6 It has also been postulated that remnants of vascular channels can persist in the inter-vertebral disc beyond the second decade of life.6
The vertebral bodies and the spinal cord, in contrast, have a fixed blood supply throughout life. One anterior and two posterior spinal arteries run longitudinally on the surface of the spinal cord from medulla to conus. They are reinforced throughout their course by transverse radicular arteries that arise from the vertebral, inter-costal and lumbar arteries through their dorsal then spinal branches (Figure 1). The spinal branches, in addition to giving radicular branches to reinforce the longitudinal spinal arteries, they also give anterior branches to supply a large portion of the posterior aspect of the vertebral bodies.42 This common arterial supply between the spinal cord and vertebral bodies is important in understanding the postulated mechanisms of FCE (Figure 1).
Figure 1.
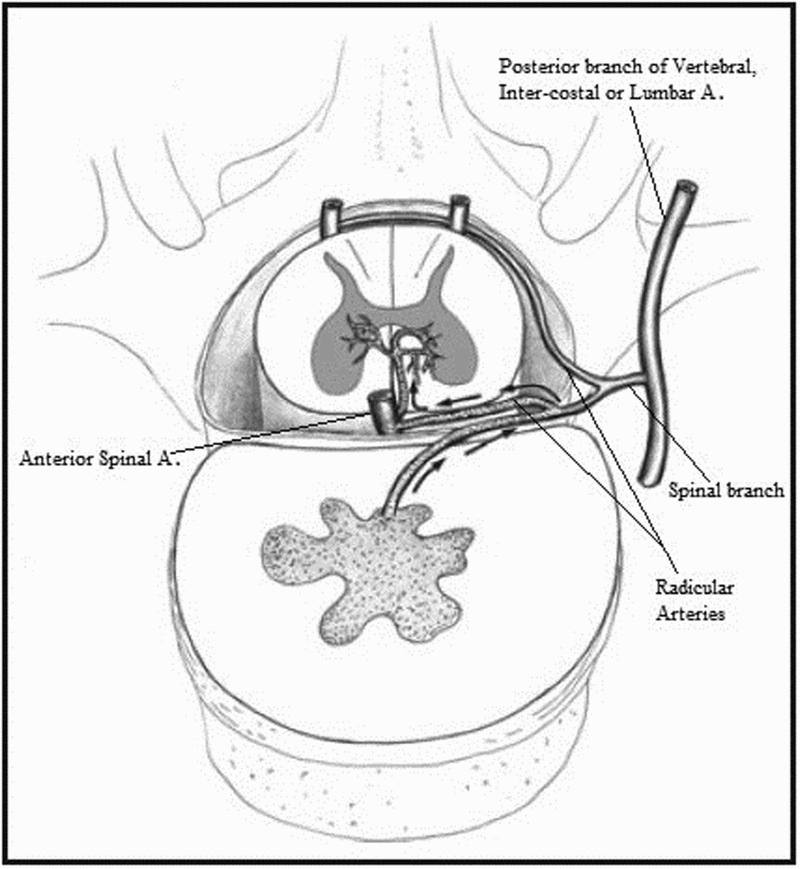
A diagram of the clinical anatomy and mechanism of FCE. Reproduced from56 with permission from John Wiley and Sons. Labels were added by the authors of this paper.
Another relevant component to the understanding of FCE mechanisms are Schmorl's nodes, which are focal masses of fibrocartilage found within the bone of vertebrae, and thus lie in close proximity to the vascular supply of the vertebral body. Schmorl's nodes are a common occurrence, present in 38% to 79% of the adult population43, 44 and are thought to have developed due to herniation of nucleus pulposus material into the body of the adjacent vertebra as a consequence of degenerative disc changes.
Mechanisms
The fibrocartilaginous disc material gains vascular access via any of three pathways: (1) revascularization of the inter-vertebral disc by normal aging or degenerative disc disease especially herniation;6,7 (2) formation of Schmorl's nodes;43,44 or (3) persistence of inter-vertebral disc vasculature into adulthood.45 It is postulated that the initial trigger for break off of fibrocartilaginous nucleus pulposus material is increased intra-disc or intra-vertebral body pressure by axial loading forces applied to the spine, such as heavy lifting, straining, falls or minor traumatic events to the neck and back.
Once in the vasculature, the fibrocartilaginous embolus can enter either an arterial or venous route to reach the spinal cord.
Arterial route
The fibrocartilaginous material travels retrograde through the arterial system supplying the spinal column, to reach the radicular artery which carries it into the spinal cord arterial system in a normal anterograde fashion (see Figure 1). This is supported by cases in which fibrocartilaginous material is found in the adjacent radicular arteries to the cord infarction.35
Venous route
The fibrocartilaginous material gain access to the venous system of the spinal column and travel initially in a normal anterograde fashion where they would enter the caval system, but then travel retrograde to the venous plexus of Batson and the parenchyma of the spinal cord. In his original article to explain metastatic lesions to the spine from breast, prostate and other cancers, Batson46 demonstrated this retrograde flow in both animal and cadaveric models, especially in the setting of increased intra-thoracic or intra-abdominal pressures which promotes reversal of venous flow away from the heart. To support this theory, Kepes et al15 demonstrated India ink in the spinal cord veins after it was injected into the vertebral body.
The initial retrograde travel in the arterial route and the eventual retrograde travel in the venous route is postulated to be aided by concomitant increases in the intra-thoracic or intra-abdominal pressure as may occur with lifting, straining, coughing or other activities associated with Valsalva maneuver. The presence of both arterial and venous FCE in some cases can be explained by either concomitant arterial and venous embolization or arterio-venous shunts that are normally present in the epidural space.47
Epidemiology and Clinical Picture
In our review of tissue confirmed cases of FCE, there was some female predominance (63.5% female vs 36.5% male). The youngest patient was 14-years-old, the oldest 78 years, the average age was 41years (SD 20 years), there was no obvious age group predominance but worth mentioning is that nearly half of the patients were under 40 years of age (n = 20, 49%).
The typical clinical picture, as that of any spinal cord infarction, is the onset of dull transient neck or back pain followed by or accompanying a syndrome of myelopathy. This classically involves a sensory level, bladder and/or bowel dysfunction and paraplegia in case of thoraco-lumbar cord disease or quadriplegia with or without respiratory compromise in case of cervical cord disease. The characteristic finding of spinal cord infarction versus inflammatory cord disease is a rapid course of symptoms to nadir, typically over hours. A pathognomonic clinical finding for anterior spinal artery infarction is the sparing of proprioception and vibratory sensation below the sensory level. A characteristic clinical symptom that may point to FCE as a cause for this spinal cord infarction is a temporal correlation with a minor or even unnoticed incident that triggers the increased intra-disc or intra-vertebral body pressure as described above in the “Mechanisms” section. In our review of tissue diagnosed FCE, 61% of the cases presented following such an event. The duration between this trigger event and the onset of symptoms varied from minutes to days, but averaged at 2.4 days. The weakness was asymmetric in 15% of the cases. There was associated neck or back pain in 76%. Nearly 40% of deaths were due to preventable respiratory complications (pulmonary embolism 20%, pneumonia 17%, aspiration 2%). Further details are outlined in Tables 2 and 3.
Table 2.
Clinical and imaging characteristics
| Characteristics | Value |
|---|---|
| Temporal Relation to a Suspected Trigger n (%) | 25 (61) |
| Mean, Standard Deviation (in days) for: | |
| Time from Trigger to Symptom Onset | 2.4, 5.7 |
| Time from Symptom Onset to Nadir | 1.3, 4 |
| Time from Symptom Onset to Death | 126, 401 |
| Characteristic Clinical Picture, n (%) | |
| Neck or Back Pain | 31 (76) |
| Sparing of Vibration or Proprioception | 6 (15) |
| Vascular Risk Factors, n (%) | |
| Hypertension | 10 (24) |
| Diabetes mellitus | 4 (10) |
| Active Smoking | 2 (5) |
| Peripheral Arterial Disease | 1 (2) |
| Age > 60 years | 1 (2) |
| Prior Stroke | 0 (0) |
| Two Vascular Risk Factors | 4 (10) |
| Three Vascular Risk Factors | 0 (0) |
| Degenerative Disc Disease (DDD), n (%) | |
| Total | 13 (32) |
| At the site of lesion | 4 (10) |
| Not at the site of lesion | 9 (22) |
| Schmorl's Node (SN), n (%) | 7 (17) |
| DDD or SN, n (%) | 16 (40) |
| Distribution of Infarction, n (%) | |
| Medulla Oblungata | 7 (17) |
| Cervical | 25 (61) |
| Thoracic | 23 (56) |
| Lumbar | 12 (29) |
| Conus | 6 (15) |
| CSF Analysis Reported, n (%) | 33 (80) |
| Normal, n (% of cases with reported CSF analysis) | 26/33 (79) |
| ↑ Protein, n (%) | 6/33 (18) |
| ↑ Cells and Myelin Based Protein, n (%) | 1/33 (3) |
| MRI Reported, n (%) | 11 |
| Unremarkable Initial MRI 35, 36, 38 | 3 (27) |
| Gadolinium Enhancement MRI34, 39 | 2 (18) |
Table 3.
Schematic approach to diagnosing FCE
| Step 1-Establish the clinical syndrome of myelopathy, sensory level being most useful.48, 49 |
| Step 2-Exclude traumatic and compressive etiologies of myelopathy by history and imaging using spine CT or MRI with and without contrast. |
Step 3-Exclude inflammatory etiologies of myelopathy; Mainly48, 49 by;
|
| Step 4-Establish the diagnosis of spinal cord infarction. This requires the above (Steps 1–3) plus one “Major” criterion or two “Minor” Criteria; |
Major Criteria:
|
Minor Criteria:
|
Step 5- Establish the high likelihood of FCE. This requires the absence of other more common etiologies of spinal cord infarction, mainly being aortic pathologies s,54, 55plus the presence of one or more of the following;
|
Diagnosis
Currently FCE is diagnosed on clinical grounds, and confirmed only with biopsy for histo-pathologic analysis (see Figure 3), usually at autopsy with one exception in the literature.34 Spinal cord infarction due to FCE and any other etiology is often mistaken for inflammatory cord lesions which are more common and more treatable. High likelihood for a clinical diagnosis of FCE is established with the above described clinical presentation along with suggestive CSF analysis and spine MRI findings. In spinal cord infarction, regardless of the cause, CSF analysis can be normal but usually shows elevated protein. It is different from inflammatory cord lesions in that it does not show pleocytosis or increased IgG index.48,49 MRI of the cord in spinal cord infarctions typically show T2 hyper-intense lesions in a vascular distribution, and unlike those of an inflammatory cord lesion, they typically do not enhance with gadolinium and also can be delayed for 12–48 hours from symptom onset.50 They can also be pathognomonicaly associated with similar radiologic changes in the posterior aspect of the opposing vertebral body,51 due to a common blood supply. In FCE, these spinal cord MRI findings are often times opposite a Schmorl's node or a disc protrusion (Figure 2). For a comprehensive approach to diagnose FCE clinically, a step-wise scheme is provided (Table 3).
Figure 3.
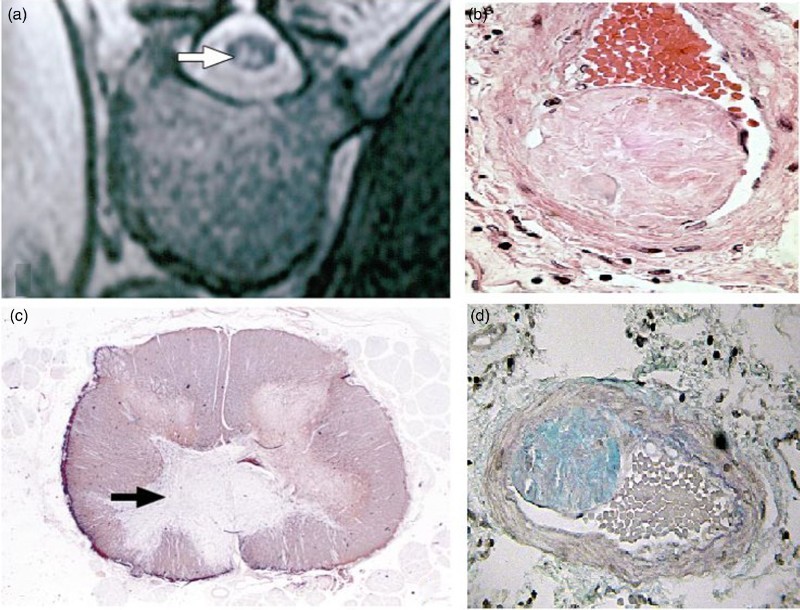
(a) Axial T2 sequence MRI showing hyper-intense lesions in the distribution of the Anterior spinal artery secondary to FCE37 in a 60-year-old woman. (b) Gross pathology for the same lesion after autopsy. (c) FCE in the anterior spinal artery, Hematoxylin and eosin staining. (d) FCE in the anterior spinal artery, Alcian staining. (a) and (b) are reproduced from37 with permission from Archives of Pathology & Laboratory Medicine. (c) and (d) are reproduced from40 with permission from Elsevier.
Figure 2.
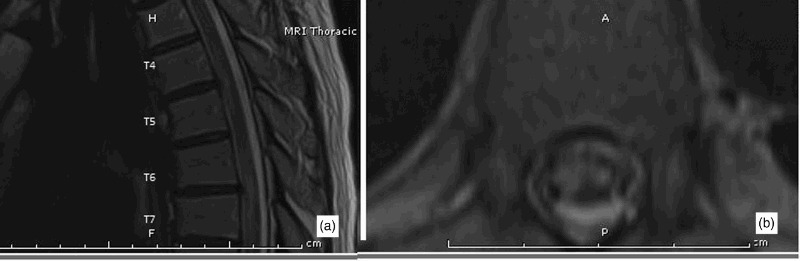
Sagital (a) and axial (b) T2 sequence MRIs showing hyper-intense lesions in the distribution of the Anterior spinal artery in a 63-year-old man clinically diagnosed with spinal cord infarction due to FCE. The lesions are characteristically opposite to disc protrusions at T4–5 and T6–7 thoracic levels.
Treatment
To date there are no available specific treatment options. Treatment primarily targets the prevention of complications and improvement of quality of life with pharmacologic and physical therapy. Throughout the literature patients have received oral and intravenous steroids, usually with the thought of treating transverse myelitis, with no improvement. Future research may target the development of intra-venous chondrolytic or fibrinolytic therapy to dissolve the FCE. This can be given empirically upon the clinical suspicion of FCE in the acute setting in an attempt to preserve spinal cord integrity before the ischemic injury occurs, much like thrombolytics are used for acute ischemic strokes of the brain.
Conclusions
Historically FCE is referred to as one of the rare causes of spinal cord infarction. Some clinicians believe this may not be true, but rather it may be under-diagnosed due to vagueness regarding its diagnosis in the clinical setting. A recent study3 published in 2011 retrospectively reviewed 164 cases that were given a diagnosis of spinal cord infarction and found that 9 (5.5%) met inclusion criteria for high likelihood of FCE. We believe this also may be an underestimation for the following reasons;
-
•
The aforementioned study3 did not include cases given the diagnosis of idiopathic transverse myelitis (TM) to confirm that they all indeed met the criteria for TM.
-
•
In clinical practice, most cases of clinical sensorimotor spinal cord dysfunction but unclear etiology, are given a diagnosis of idiopathic transverse myelitits (TM) or myelopathy. A large portion of these cases may be due to FCE, specifically those with no signs of inflammation in the CSF. This is highly likely given that MRI T2 lesions in 33% of cases with spinal cord infarctions do not follow an arterial territory53 and thus are indistinguishable from TM.
-
•
Several of the autopsy confirmed FCE cases were initially presumed to be idiopathic transverse myelitis, even in the absence of inflammatory signs in the CSF.15,17,24,36,38,39
-
•
Despite the rarity of the overall reported cases in the literature, there are several incidents where multiple tissue confirmed cases are reported by the same team.10,16–18,27,28,32 This supports the theory suggesting that when a clinical familiarity with the diagnosis of FCE is present, it is more commonly and accurately diagnosed.
-
•
There have been reports of incidentally found FCE in the spinal cord vasculature on autopsy.10
In contrast, other clinicians argue that there is no evidence that these cases of idiopathic myelopathies are indeed spinal cord infarctions due to FCE, furthermore the empiric treatment of inflammatory myelopathy with a short course of intravenous steroids is relatively safe and the benefit largely outweighs the risks, even in cases of uncertain inflammatory myelopathy. Others argue that there is also no evidence against this theory of under-diagnosing FCE. If further research is performed to acutely reverse this condition by chondrolytic therapy, then this notion becomes important. To date there has been no large study that revised cases with established diagnoses of idiopathic TM or myelopathy to rule out that these may be spinal cord infarctions due to FCE. Until this study is performed, it may be reasonable to assume that FCE is more common than reported as this will open the door for research into safe acute medical interventions that can be offered to cases suspicious for FCE upon their presentation, as described above in the “Treatment” section.
Acknowledgments
Special thanks to Shannon Taylor and Diane Schwartz for providing the necessary articles. Also, thanks to Sherif Abdelrazek, Howida Omar and AbdelMageed Elsadek for their help in the translation of foreign articles.
ORCID
Mahmoud A. AbdelRazek 0000-0002-2391-3502
References
- 1.Naiman JL, Donohue WL, Prichard JS. Fatal nucleus pulposus embolism of spinal cord after trauma. Neurology 1961;11:83–7. doi: 10.1212/WNL.11.1.83 [DOI] [PubMed] [Google Scholar]
- 2.Schreck RI, Manion WL, Kambin P, Sohn M. Nucleus pulposus pulmonary embolism. A case report. Spine (Phila Pa 1976) 1995;20(22):2463–6. doi: 10.1097/00007632-199511001-00016 [DOI] [PubMed] [Google Scholar]
- 3.Mateen FJ, Monrad PA, Hunderfund AN, Robertson CE, Sorenson EJ. Clinically suspected fibrocartilaginous embolism: clinical characteristics, treatments, and outcomes. Eur J Neurol 2011;18(2):218–25. doi: 10.1111/j.1468-1331.2010.03200.x [DOI] [PubMed] [Google Scholar]
- 4.Masson C, Boukriche Y, Berthelot JL, Colombani JM. Vertebra, rib and spinal cord infarction caused by probable fibrocartilaginous embolism. Cerebrovasc Dis 2001;12(2):142–3. doi: 10.1159/000047694 [DOI] [PubMed] [Google Scholar]
- 5.De Risio L, Platt SR. Fibrocartilaginous embolic myelopathy in small animals. Vet Clin North Am Small Anim Pract 2010;40(5):859–69. doi: 10.1016/j.cvsm.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 6.Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg 2006;88 Suppl 2:10–4. doi: 10.2106/JBJS.F.00019 [DOI] [PubMed] [Google Scholar]
- 7.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976) 2002;27(23):2631–44. doi: 10.1097/00007632-200212010-00002 [DOI] [PubMed] [Google Scholar]
- 8.Laterre Syndrome Spinal Anterieur par Embolies Multiples de tissu Fibro-cartilagineux. Revue Neurologique 1962;106(106):685–90. [Google Scholar]
- 9.Ho KL, Gorell JM, Hayden MT. Fatal spinal cord infarction caused by fibrocartilaginous embolization of the anterior spinal artery. Hum Pathol 1980;11(5):471–5. doi: 10.1016/S0046-8177(80)80056-6 [DOI] [PubMed] [Google Scholar]
- 10.Feigin I, Popoff N, Adachi M. Fibrocartilaginous venous emboli to the spinal cord with necrotic myelopathy. J Neuropathol Exp Neurol 1965;24:63–74. doi: 10.1097/00005072-196501000-00006 [DOI] [PubMed] [Google Scholar]
- 11.Bodechtel G. On the differential diagnostic difficulties in the border fields between internal medicine and neurology. Munch Med Wochenschr 1968;110(16):969–80. [PubMed] [Google Scholar]
- 12.Bruno MS, Ober WB. Sudden onset of severe chest pain and paralysis. N Y State J Med 1969;69(3):446–54. [PubMed] [Google Scholar]
- 13.Lyvovskii AM. Embolism of spinal cord vessels by intervertebral disk tissue. Zh Nevropatol Psikhiatr Im S S Korsakova 1969;69(8):1151–7. [PubMed] [Google Scholar]
- 14.Jurkovic I, Eiben E. Fatal myelomalacia caused by massive fibrocartilaginous venous emboli from nucleus pulposus. Acta Neuropathol 1970;15(3):284–7. doi: 10.1007/BF00686774 [DOI] [PubMed] [Google Scholar]
- 15.Kepes JJ, Reynard JD. Infarction of spinal cord and medulla oblongata caused by fibrocartilaginous emboli. Report of a case Virchows Arch A Pathol Pathol Anat 1973;361(3):185–93. doi: 10.1007/BF00543983 [DOI] [PubMed] [Google Scholar]
- 16.Hubert JP, Ectors M, Ketelbant-Balasse P, Flament-Durand J. Fibrocartilaginous venous and arterial emboli from the nucleus pulposus in the anterior spinal system. A clinicopathological observation. Eur Neurol 1974;11(3):164–71. doi: 10.1159/000114315 [DOI] [PubMed] [Google Scholar]
- 17.Bots GT, Wattendorff AR, Buruma OJ, Roos RA, Endtz LJ. Acute myelopathy caused by fibrocartilaginous emboli. Neurology 19;31(10):1250–6. doi: 10.1212/WNL.31.10.1250 [DOI] [PubMed] [Google Scholar]
- 18.Hubert JP, Retif J, Brihaye J, Flament-Durand J. Spinal infarction due to nucleus pulposus emboli. Acta Neurol Belg 1974;74(5):297–305. [PubMed] [Google Scholar]
- 19.Roitzsch E. Fibrocartilaginous embolia of the spinal cord - a rare cause of myelomalacia (author's transl). Zentralbl Allg Pathol 1975;119(1–2):100–3. [PubMed] [Google Scholar]
- 20.Peiffer J, Wenig C, Mausle E. Acute paraplegia due to embolism from nucleus pulposus tissue (author's transl). Dtsch Med Wochenschr 1976; 101(15):583–6. doi: 10.1055/s-0028-1104118 [DOI] [PubMed] [Google Scholar]
- 21.Hanski W, Rydzewska M, Fundowicz R. Emboli of spinal cord vessels with tissue of nucleus pulposus. Neuropatol Pol 1977;15(4):479–90. [PubMed] [Google Scholar]
- 22.Schairer E, von Albert HH. Ascending tetraplegia after embolic occlusion of medullary arteries by nucleus pulposus tissue (author's transl). MMW Munch Med Wochenschr 1977;119(44):1433–6. [PubMed] [Google Scholar]
- 23.Budka H, Perneczky A, Pusch S. Infarction of the spinal cord in the posterior spinal arterial supply area as a result of intervertebral disc embolism (author's transl). Wien Klin Wochenschr 1979;91(17):578–83. [PubMed] [Google Scholar]
- 24.Toro G, Roman GC, Navarro-Roman L, Cantillo J, Serrano B, Vergara I. Natural history of spinal cord infarction caused by nucleus pulposus embolism. Spine (Phila Pa 1976) 1994;19(3):360–6. doi: 10.1097/00007632-199402000-00020 [DOI] [PubMed] [Google Scholar]
- 25.Srigley JR, Lambert CD, Bilbao JM, Pritzker KP. Spinal cord infarction secondary to intervertebral disc embolism. Ann Neurol 1981;9(3):296–301. doi: 10.1002/ana.410090315 [DOI] [PubMed] [Google Scholar]
- 26.Kase CS, Varakis JN, Stafford JR, Mohr JP. Medial medullary infarction from fibrocartilaginous embolism to the anterior spinal artery. Stroke 1983;14(3):413–8. doi: 10.1161/01.STR.14.3.413 [DOI] [PubMed] [Google Scholar]
- 27.Barz H, Majerowitsch B. Myelomalacia caused by embolism of the substantia gelatinosa in the vertebral column and spinal cord arteries. Zentralbl Allg Pathol 1986;131(2):119–25. [PubMed] [Google Scholar]
- 28.Barz H, Hackebeil C. Cartilage tissue embolism as a cause of myelomalacia. A case report and review of the literature. Pathologe 1989;10(5):300–5. [PubMed] [Google Scholar]
- 29.Banerjee AK, Deodhar SD. Cartilage embolism of spinal cord. J Neurol Neurosurg Psychiatry 1989;52(10):1201–2. doi: 10.1136/jnnp.52.10.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kestle JR, Resch L, Tator CH, Kucharczyk W. Intervertebral disc embolization resulting in spinal cord infarction. Case report. J Neurosurg 1989;71(6):938–41. [DOI] [PubMed] [Google Scholar]
- 31.Bockenek WL, Bach JR, Alba AS, Cravioto HM. Fibrocartilaginous emboli to the spinal cord: a case report. Arch Phys Med Rehabil 1990;71(10):754–7. [PubMed] [Google Scholar]
- 32.Skully R, Mark E, McNeely W. Case records of Massachusetts General Hospital. Case 5. New Engl J Med 1991;324:322–32. doi: 10.1056/NEJM199101313240508 [DOI] [PubMed] [Google Scholar]
- 33.Moorhouse DF, Burke M, Keohane C, Farrell MA. Spinal cord infarction caused by cartilage embolus to the anterior spinal artery. Surg Neurol 1992;37(6):448–52. doi: 10.1016/0090-3019(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 34.Mikulis DJ, Ogilvy CS, McKee A, Davis KR, Ojeman RG. Spinal cord infarction and fibrocartilagenous emboli. AJNR Am J Neuroradiol 1992;13(1):155–60. [PMC free article] [PubMed] [Google Scholar]
- 35.Yousef OM, Appenzeller P, Kornfeld M. Fibrocartilagenous embolism: an unusual cause of spinal cord infarction. Am J Forensic Med Pathol, 1998. 19(4):395–9. doi: 10.1097/00000433-199812000-00020 [DOI] [PubMed] [Google Scholar]
- 36.Freyaldenhoven TE, Mrak RE, Rock L. Fibrocartilaginous embolization. Neurology 2001;56(10):1354. doi: 10.1212/WNL.56.10.1354 [DOI] [PubMed] [Google Scholar]
- 37.Alexander RT, Cummings TJ. Pathologic quiz case: acute-onset paraplegia in a 60-year-old woman. Spinal cord infarction secondary to fibrocartilaginous (intervertebral disk) embolism. Arch Pathol Lab Med 2003;127(8):1047–8. [DOI] [PubMed] [Google Scholar]
- 38.Uppal S, Dash S, Sharer L, Clark Lambert W, Heller DS, Pullicino P. Spinal cord infarction secondary to nucleus pulposus embolization in pregnancy. Mod Pathol 2004;17(1):121–4. doi: 10.1038/modpathol.3800037 [DOI] [PubMed] [Google Scholar]
- 39.Duprez TP, Danvoye L, Hernalsteen D, Cosnard G, Sindic CJ, Godfraind C. Fibrocartilaginous embolization to the spinal cord: serial MR imaging monitoring and pathologic study. AJNR Am J Neuroradiol 2005;26(3):496–501. [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer HJ, Monticelli F, Kiesslich J. Fatal embolism of the ASA after local cervical analgetic infiltration. Forensic Sci Int 2005;149(2–3):115–9. doi: 10.1016/j.forsciint.2004.05.018 [DOI] [PubMed] [Google Scholar]
- 41.Piao YS, Lu DH, Su YY, Yang XP. Anterior spinal cord infarction caused by fibrocartilaginous embolism. Neuropathology 2009;29(2):172–5. doi: 10.1111/j.1440-1789.2008.00938.x [DOI] [PubMed] [Google Scholar]
- 42.Ratcliffe JF. The arterial anatomy of the developing human dorsal and lumbar vertebral body. A microarteriographic study. J Anat 1981;133(Pt 4):625–38. [PMC free article] [PubMed] [Google Scholar]
- 43.Hilton RC, Ball J, Benn RT. Vertebral end-plate lesions (Schmorl's nodes) in the dorsolumbar spine. Ann Rheum Dis 1976;35(2):127–32. doi: 10.1136/ard.35.2.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kyere KA, Than KD, Wang AC, Rahman SU, Valdivia-Valdivia JM, La Marca F, et al. Schmorl's nodes. Eur Spine 2012;21(11):2115–21. doi: 10.1007/s00586-012-2325-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roshal D, Gutierrez C, Brock D, Kremens D. Pearls & Oy-sters: fibrocartilaginous embolism myelopathy. Neurology 2010;74(7):e21–3. doi: 10.1212/WNL.0b013e3181cff6e9 [DOI] [PubMed] [Google Scholar]
- 46.Batson OV. The function of the vertebral veins and their role in the spread of metastases. Ann Surg 1940;112(1):138–49. doi: 10.1097/00000658-194007000-00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vuia O, Alexianu M. Arteriovenous shunt in the spinal cord circulation. Acta Neurol Scand 1969;45(2):216–23. doi: 10.1111/j.1600-0404.1969.tb01233.x [DOI] [PubMed] [Google Scholar]
- 48.Frohman EM, Wingerchuk DM. Clinical practice. Transverse myelitis. N Engl J Med 2010;363(6):564–72. doi: 10.1056/NEJMcp1001112 [DOI] [PubMed] [Google Scholar]
- 49.Consortium T. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 2002;59(4):499–505. doi: 10.1212/WNL.59.4.499 [DOI] [PubMed] [Google Scholar]
- 50.Reisner A, Gary MF, Chern JJ, Grattan-Smith JD. Spinal cord infarction following minor trauma in children: fibrocartilaginous embolism as a putative cause. J Neurosurg Pediatr 2013;11(4):445–50. doi: 10.3171/2013.1.PEDS12382 [DOI] [PubMed] [Google Scholar]
- 51.Amoiridis G, Ameridou I, Mavridis M. Intervertebral disk and vertebral body infarction as a confirmatory sign of spinal cord ischemia. Neurology 2004;63(9):1755. doi: 10.1212/01.WNL.0000142973.33952.68 [DOI] [PubMed] [Google Scholar]
- 52.Nedeltchev K, Loher TJ, Stepper F, Arnold M, Schroth G, Mattle HP, et al. Long-term outcome of acute spinal cord ischemia syndrome. Stroke 2004;35(2):560–5. doi: 10.1161/01.STR.0000111598.78198.EC [DOI] [PubMed] [Google Scholar]
- 53.Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol 2006;63(8):1113–20. doi: 10.1001/archneur.63.8.1113 [DOI] [PubMed] [Google Scholar]
- 54.Blakemore WF, Palmer AC. Delayed infarction of spinal cord white matter following x-irradiation. J Pathol 1982;137(4):273–80. doi: 10.1002/path.1711370402 [DOI] [PubMed] [Google Scholar]
- 55.Cheshire WP, Santos CC, Massey EW, Howard JF Jr. Spinal cord infarction: etiology and outcome. Neurology 1996;47(2):321–30. doi: 10.1212/WNL.47.2.321 [DOI] [PubMed] [Google Scholar]
- 56.Heckmann JG, Dutsch M, Struffert T, Dorfler A, Schwab S. Spinal cord infarction: a case of fibrocartilaginous embolism? Eur J Neurol 2007;14(8):e23–4. doi: 10.1111/j.1468-1331.2007.01876.x [DOI] [PubMed] [Google Scholar]


