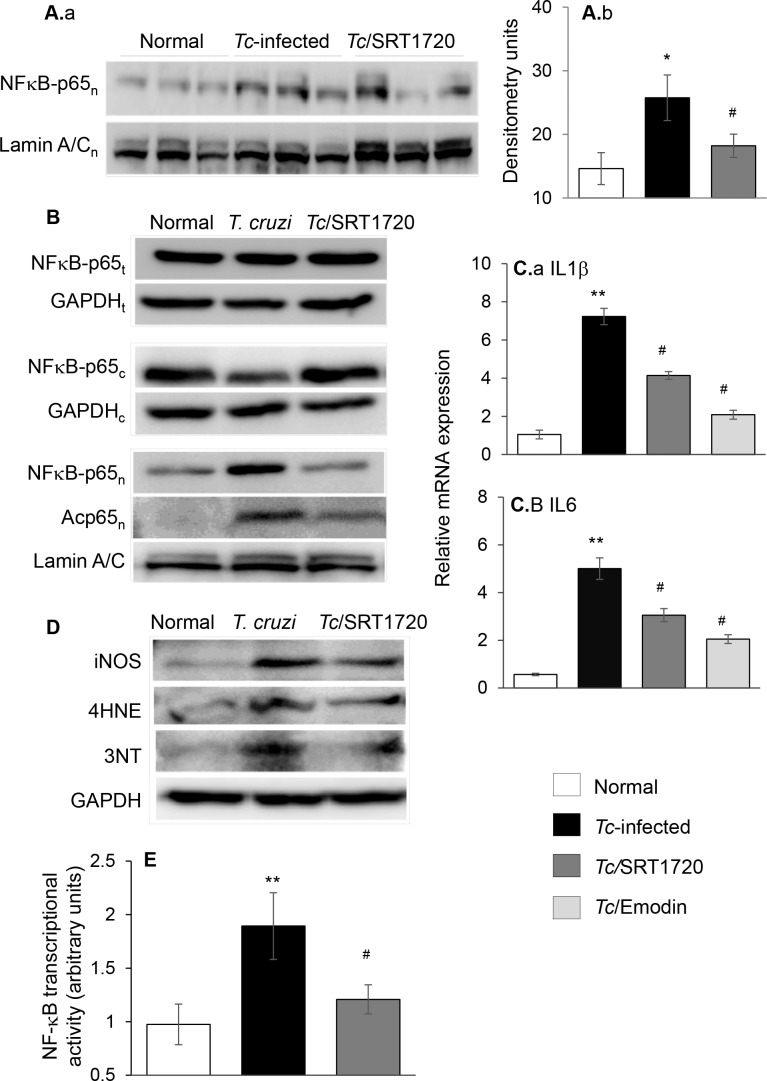
Fig 7. T. cruzi induced NF-κB transcriptional activity (± SRT1720).
T. (A) Mice were Tc-infected, SRT1720-treated, and harvested at 150 days pi. Nuclear fractions from heart homogenates were prepared and submitted to Western blot analysis for NFκB p65 subunit and Lamin A/C (panel a). Densitometry analysis of NFκB p65 band, normalized to Lamin A/C, is shown in panel b. (B) Cardiac myocytes were infected with T. cruzi and incubated in presence or absence of 1 μM SRT1720 for 24 h. Total homogenates, and cytosolic and nuclear fractions were prepared as described in Materials and Methods. Shown are representative immunoblots for NFκB p65 subunit (total, cytosolic, and nuclear), and acetylated-p65 (nuclear) levels. Lamin A/C (nuclear fractions) and GAPDH (cytosolic and total homogenates) were analyzed for loading control. (C) Real time qRT-PCR measurement of mRNA levels for (a) IL1β and (b) IL6 in cardiomyocytes that were infected with T. cruzi and treated with SRT1720 (1 μM) or emodin (NFκB antagonist, 50 μM) for 24 h. Fold change was determined after normalizing the data with GAPDH mRNA. (D) Representative immunoblots for iNOS, 4-HNE and 3-NT (GAPDH control) levels in cell homogenates are shown. (E) NFκB transcriptional activity. HEK293 cells were transiently transfected with NFκB-TATA-luciferase reporter plasmid. Cells were co-transfected with a Renilla luciferase plasmid for normalization of transfection efficiency. Transfected cells were infected (cell: Tc ratio, 1:3) and incubated for 24 h in presence or absence of SRT1720. The relative NFκB transcriptional activity was measured by using a Dual Luciferase Assay System and normalized to Renilla luciferase activity. Bar graphs show mean value ± SD derived from triplicate experiments (3 replicates per group per experiment). Significance is shown as *,#p<0.05, **,##p<0.01, ***,###p<0.001 (*normal control vs. Tc-infected, # Tc-infected vs. Tc-infected and SRT1720- or emodin-treated).