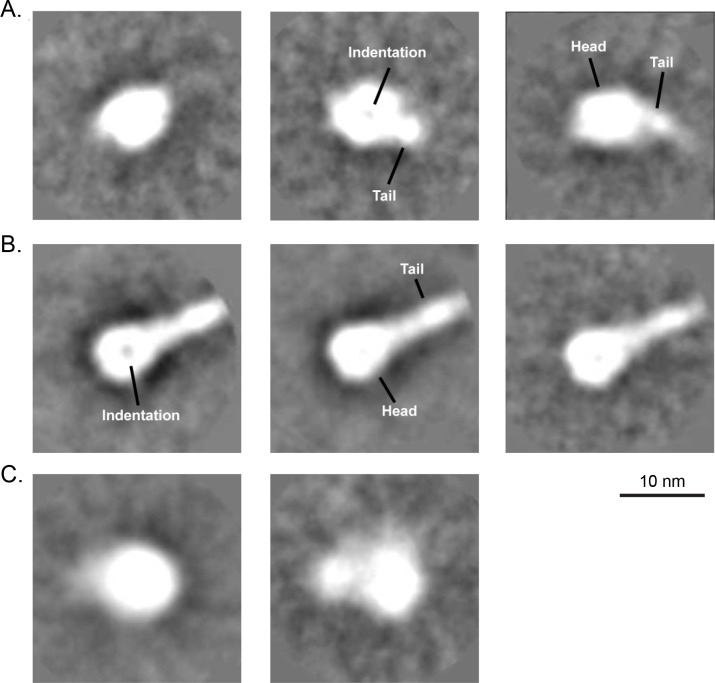
Fig 5. Analysis of RSV F proteins by negative stain transmission electron microscopy and 2D class averaging.
(A) Representative averages for DS-Cav1 stored at -70°C and thawed immediately prior to analysis, indicating that mostly globular particles (left) or particles that contained a slightly tapered, short tail portion (middle and right) were observed. A characteristic indentation was visible in some of the head domains (middle). (B) Representative averages for postfusion RSV F protein stored at -70°C and thawed immediately prior to analysis, indicating that particles with distinct head and tail portions were primarily observed. A characteristic indentation was also visible in some of the head domains (left). (C) Representative averages for DS-Cav1 protein after long-term storage at 4°C. The lack of detail of the averages suggests that the conformation of 4°C-stored DS-Cav1 is more heterogeneous and does not resemble the postfusion form.