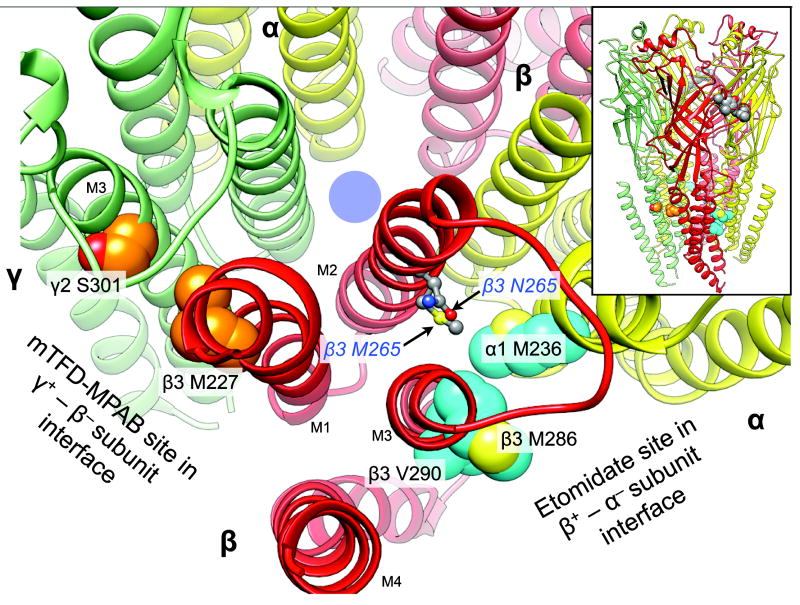
Figure 1.
The location of the N265M mutation relative to that of the etomidate and R–mTFD-MPAB sites on a homology model of α1β3γ2L GABAARs. The backbone of the receptor is represented as ribbons with relevant residues in space filled mode. The five subunits of the GABAAR are arranged around a central ion–conducting pore (blue circle) and colored as follows: α1, yellow; β3, red, and γ2 green. The main figure shows a cross section of the transmembrane domain with parts of the extracellular domain removed for clarity. Residues photolabeled by etomidate derivatives or R–mTFD-MPAB are shown with cyan and orange carbons respectively. Other atoms are colored conventionally (carbons grey; oxygens red; nitrogens blue, and sulfurs yellow). The residues at β3 265 are represented as ball and stick. The inset at top right shows the whole pentamer with a cluster of space filled agonist–associated residues in the extracellular domain in the β3+ – α1− subunit interface (α1 Phe-65; β3 Tyr-157 & -205, Phe-200). The homology model is based on the GluCl chloride channel (3RHW.pdb) (11,22). The intracellular domain is not modeled because no structural information is available.