Abstract
Transcranial focused ultrasound (FUS) can noninvasively transmit acoustic energy with a high degree of accuracy and safety to targets and regions within the brain. Technological advances, including phased array transducers and real-time temperature monitoring with magnetic resonance (MR) thermometry, have created new opportunities for FUS research and clinical translation. Neuro-oncology, in particular, has become a major area of interest, as FUS offers a multifaceted approach to the treatment of brain tumors. FUS has the potential to (1) generate cytotoxicity within tumor tissue, both directly via thermal ablation and indirectly through radiosensitization and sonodynamic therapy; (2) enhance the delivery of therapeutic agents to brain tumors by transiently opening the blood-brain barrier and/or improving distribution through the brain extracellular space; and (3) modulate the tumor microenvironment in order to generate an immune response. In this review, we describe each of these applications for FUS, the proposed mechanisms of action, and the preclinical and clinical studies that have set the foundation for utilizing FUS in neuro-oncology.
Keywords: Blood-brain barrier, brain neoplasms, drug delivery, extracellular space, high-intensity focused ultrasound, immunomodulation
INTRODUCTION
Transcranial focused ultrasound (FUS) has been investigated for over 60 years, with seminal studies by John Lynn and Tracy Putnam in the 1940s and William and Francis Fry in the 1950s establishing the potential of applying ultrasound to cerebral tissue.1–3 Early explorations of FUS for clinical use, however, were limited by the need for a craniectomy due to beam distortion and energy absorption by the intact skull. In the 1990s, hemispheric phased arrays of transducers were developed along with software that corrects for the phase aberrations produced by the variable thickness of the skull. This revolutionized the field by allowing for the noninvasive transmission of ultrasound beams to focal regions across irregular bone and tissue interfaces.4 Additionally, advances in magnetic resonance (MR) imaging, specifically the development of MR thermometry, allowed temperature changes to be visualized in real time, thereby enabling safety monitoring and confirmation of the energy being applied at the acoustic focus.5
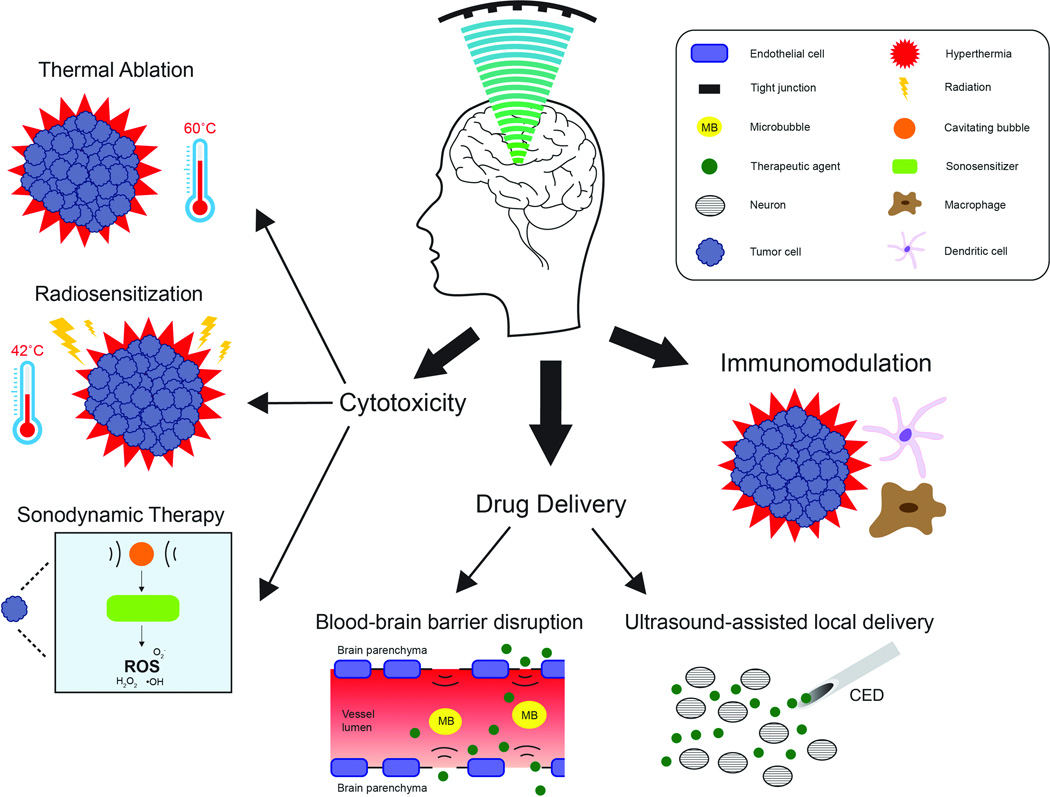
Once these technologies were incorporated, FUS research in the neurosciences increased dramatically, building on prior successes in treating non-neurological disorders such as uterine fibroids. Ultimately, the existing clinical MR-guided focused ultrasound (MRgFUS) devices were modified for the non-invasive application of focused exposures in the human brain. MRgFUS recently completed a clinical trial for the treatment of essential tremor (NCT01304758) and is currently being evaluated for Parkinson’s disease (NCT01772693, NCT02246374, NCT02263885), depression (NCT02348411), epilepsy (NCT02151175), neuropathic pain (NCT01699477), and acute brain injury (NCT02522429). The technology has numerous other potential therapeutic applications, including the treatment of stroke, obsessive compulsive disorder, Alzheimer’s disease, trigeminal neuralgia, and hydrocephalus (for a review, see Medel et al6). Neuro-oncology, in particular, is receiving renewed attention, as MRgFUS provides new options for the targeted, noninvasive treatment of brain tumors, in contrast to more invasive thermal approaches that are emerging as viable treatment options.7,8 This article reviews the preclinical and clinical work exploring MRgFUS for (1) generating cytotoxicity within tumor tissue, (2) enhancing the delivery or activity of therapeutic agents, and (3) modulating the tumor microenvironment to enhance immune recognition and clearance (Figure 1 and Table 1).
Figure 1.
Applications of therapeutic ultrasound in neuro-oncology. Transcranial focused exposures may be used for thermal ablation, radiosensitization, sonodynamic therapy, BBB disruption, ultrasound-assisted local delivery, and immunomodulation. CED, convection-enhanced delivery; ROS, reactive oxygen species.
Table 1.
Applications of focused ultrasound in neuro-oncology
| Application | Rate of energy deposition |
Energy intensity |
Acoustic mechanism |
Biological effect |
|---|---|---|---|---|
| Thermal ablation | Continuous | High | Hyperthermia (high) |
Coagulative necrosis |
| Radiosensitization | Continuous | Low | Hyperthermia (low) |
Prevents DNA repair |
| Sonodynamic therapy | Pulsed | High | Inertial cavitation | Formation of reactive oxygen species |
| Blood-brain barrier opening |
Pulsed | Low | Stable cavitation | Disruption of tight junctions |
| Enhanced local delivery | Pulsed | Low or High | Radiation forces | Tissue displacement and shear forces |
| Immunomodulation | Continuous and Pulsed |
High | Miscellaneous a | Miscellaneous a |
Ultrasound-mediated immunomodulation may occur due to thermal or mechanical exposures, with the latter being more prominent. The result is the presentation of tumor-specific antigens to antigen presenting cells, changes in chaperone expression and cytokine secretion.
BIOLOGICAL EFFECTS OF ULTRASOUND—MECHANISMS OF ACTION
Heat
FUS utilizes either spherically curved transducers or phased arrays of smaller single-element transducers to focus energy into a small volume at a particular distance from the transducer. At the focal zone, the spatial intensity is several orders of magnitude higher than in the pre-focal region. As a result, the effects of the ultrasound beam are generally limited to the focal point, where the high rate of energy deposition results in the efficient generation of heat and subsequent temperature elevations that can reach up to 60°C in seconds (for a comprehensive review, see Haar and Coussios9). For the generation of thermal lesions, these can be very sharply delineated from a homogenous thermal dose, with lethal and sublethal effects being separated by only several cell thicknesses.10,11 The diameter of the focal zone is typically around 1 mm, and the length can range from 5 to 20 times this dimension. These parameters are dependent on the specific dimensions of the transducer (ie, the diameter and the radius of curvature) as well as the frequency.12 Due to the small volume of the focal zone relative to the regions that are typically treated (eg, a mid-sized uterine fibroid, which is 50–100 mm3), a large number of contiguous or overlapping treatments are required, leading to potentially long treatment times.11,13
Alternatively, pulsed exposures with short duty cycles (eg, 5%–10%) reduce the overall temporal average intensity, typically generating minimal temperature elevations on the order of 4°C–5°C.14,15 Pulsed focused ultrasound (pFUS) therefore allows for the non-thermal, mechanical effects of ultrasound to predominate. These include acoustic cavitation, acoustic radiation forces, and acoustic streaming.
Acoustic Cavitation
Ultrasound is a pressure wave consisting of positive (compressive) and negative (rarefactive) components, where the negative component may enable the expansion of small, stabilized gas-filled “cavities” or bubbles within a liquid medium. As the ultrasound exposure continues, the bubbles oscillate, with their diameter varying with the alternating pressure field. The bubbles may continue to oscillate in a stable fashion, (ie, non-inertial cavitation), but if the pressure wave amplitude increases, the bubbles collapse (ie, inertial cavitation), producing violent shock waves and high-velocity jets, with substantial mechanical and potentially detrimental effects on the surrounding tissues.16
Radiation Forces and Acoustic Streaming
When an ultrasound beam transfers momentum to a reflecting or absorbing surface, a small, steady, unidirectional force is produced along the direction of the beam. These radiation forces, if large enough, produce displacements in the tissue at the focal point, though not in the surrounding tissues. The resulting non-uniformity in displacement may produce strain in the tissue.17 In a similar fashion, acoustic streaming results from the radiation forces that take place specifically within a liquid medium. The resulting circulation set up by the acoustic field may enhance convection as well as produce shear forces that can cause tissue damage.18,19
CYTOTOXICITY
Through the mechanisms described above, FUS may produce a variety of effects that directly or indirectly destroy tumor tissue. Early on, investigators realized that the temperature elevations at the focal zone can ablate tumor cells, particularly deep-seated tumors that are difficult to access surgically. These include tumors in the liver,20 prostate,21 and brain,22 as well as bone metastases.23 More recently, however, ultrasound has been proposed as an adjuvant therapy that contributes to cytotoxicity via subtler processes, including radiosensitization24 and sonodynamic therapy (SDT).25–27
Thermal Ablation
Heat deposition resulting in thermal ablation is the most direct mechanism by which FUS can be used to treat brain tumors. Continuous exposures result in high rates of energy deposition, producing progressive elevations in temperature of tens of degrees Celsius. At temperatures above 55°C, cellular death occurs as a result of coagulative necrosis, accompanied by protein denaturation and the disruption of cellular membranes.28,29
FUS is now commonly used for the ablation of uterine fibroids30 and prostate cancer.31 In a meta-analysis involving 1594 patients in 10 countries, treatment of uterine fibroids with MRgFUS resulted in moderate reductions in fibroid volume (most studies reported reductions of 10%–30%), and fibroid-associated symptom severity improved significantly.32 Similar results exist for the treatment of prostate cancer with FUS, which is carried out worldwide. Mid- and long-term progression-free survival rates following sonication are ~70%, and negative postoperative biopsies occur in 80% of cases.33,34
The treatment of brain tumors with FUS, however, has been limited to small case series (Table 2). In the early 2000s, Ram et al22 used MRgFUS to treat 3 patients with recurrent glioblastoma (GBM). Although post-treatment imaging and histopathology revealed evidence of thermocoagulation in 2 of the 3 tumors, a craniectomy was required prior to sonication in order to create an acoustic window. At the time, this prerequisite negated one of the main attractions of FUS as a tool for ablation—its noninvasive nature. Additionally, one of the patients developed a secondary focus of thermocoagulation along the sonication path, resulting in a mild left hemiparesis. As a result, the device was later modified to provide temperature measurements along the path of the ultrasound beam, to monitor for heating of the intervening, peritumoral tissue.
Table 2.
Completed and ongoing clinical trials studying focused ultrasound in neuro-oncology
| Application | Phase | Tumor Type | Patients | Adverse Effects | Outcome | Reference |
|---|---|---|---|---|---|---|
| Thermal ablation |
I | Recurrent glioblastoma |
3 |
|
Resolution of enhancement of treated volume in 2 of 3 patients |
Ram et al, 200622 |
| I | Glioblastoma | 4 |
|
Limitations in device power prevented thermal coagulation |
McDannold et al, 201035 |
|
| I/II | High grade glioma or brain metastasis |
Ongoing | - | - |
NCT01698437, NCT00147056, NCT01473485 |
|
| Radiosensitization | I | Grade III astrocytoma and glioblastoma |
15 |
|
Target temperature achieved in 41.9% of measured regions |
Guthkelch et al, 199124 |
| Sonodynamic therapy |
- | - | - | - | - | - |
| Blood-brain barrier opening |
I | Glioblastoma | 1 | Unknownb | Blood-brain barrier opening confirmed with contrast enhancement on MRIb |
NCT02343991 |
| Enhanced local delivery |
- | - | - | - | - | - |
| Immunomodulation | - | - | - | - | - | - |
Intracranial hemorrhage occurred as a result of thermocouple probe placement, and was symptomatic in 2 cases.
Recruitment of additional patients is currently ongoing. Additional information is needed before comments regarding adverse effects and outcomes can be offered.
More recently, McDannold et al35 reported their experience treating 3 patients with inoperable GBM as part of a phase I clinical trial. Although this was the first group to successfully focus an ultrasound beam through an intact cranium, the version of the device being used at the time did not provide enough power to reach the temperature threshold for coagulative necrosis. Furthermore, a fourth patient experienced an intracranial hemorrhage for unclear reasons, resulting in closure of the study.6
Currently, 3 phase I clinical trials (NCT01698437, NCT00147056, NCT01473485) are ongoing to verify the safety of the device and the feasibility of thermal ablation of brain tumors. In order to minimize the risk of intracranial hemorrhage, patients with a bleeding diathesis are currently excluded from the trials, as are those with vascular tumors. Furthermore, in order to maintain precise control over the energy deposition along the ultrasound beam path, exclusion criteria also include the presence of cystic areas adjacent to the tumor, clips or implants in the sonication path, and dural patches or skull reconstructions. Signs of intracranial hypertension or tumoral mass effect are contraindications to MRgFUS as well, given the concern that sonication may result in a transient increase in cerebral edema. These Phase I trials are a critical step toward the translation of MRgFUS into clinical practice.
Radiosensitization
Although FUS can achieve cytotoxicity directly via thermal ablation, it can also do so indirectly by sensitizing brain tumors to radiotherapy via a hyperthermia-based mechanism. Radiation is an integral part of the current standard of care for patients with GBM and other brain tumors, which commonly involves maximum safe surgical resection followed by adjuvant radiation and chemotherapy.36 Additionally, whole brain radiation therapy followed by a stereotactic radiosurgery (SRS) boost plays an important role in the management of patients with brain metastases.37,38
Non-destructive hyperthermia (to approximately 42°C) has become well established as a means of enhancing the effects of radiation therapy, with numerous randomized trials demonstrating its efficacy in a variety of tumor types.39–42 Hyperthermia is thought to sensitize cancer cells to the effects of radiation by preventing the repair of DNA damage and by inducing tumor reoxygenation.43,44 Hyperthermia preferentially affects cells in the S phase of the cell cycle, when they are most radioresistant.45 Recent evidence also suggests that hyperthermia may have a specific effect on glioma stem-like cells through the inhibition of AKT signaling.46
A randomized phase II/III study involving 79 patients with focal GBM explored the radiosensitizing effects of hyperthermia.42 Following surgical resection, patients underwent fractionated external beam radiation to a dose of 59.4 Gy with concomitant oral hydroxyurea. Patients with an “implantable” tumor at the completion of treatment were then randomized to interstitial brachytherapy with or without microwave-based hyperthermia. Hyperthermia was associated with significantly longer time to progression (P = .045) and survival from date of diagnosis (P = .02). Median survival was 76 weeks vs 85 weeks, and 2-year survival was 15% vs 31% for the control vs hyperthermia groups, respectively.
Historically, however, the challenge has been to efficiently and safely produce low-level hyperthermia within brain tissue. One of the more recent technologies to emerge has been MR-guided laser interstitial thermal therapy, which utilizes a solid-state diode laser to generate a focal region of hyperthermia. Despite accumulating evidence regarding its efficacy, the technology still requires the laser probe to be stereotactically implanted into the tumor (for a review, see Hawasli et al47).
FUS, however, represents a noninvasive alternative; utilizing the pulsed sequences described above, low levels of hyperthermia can be achieved in focal, deep brain targets. As early as 1991, FUS-induced hyperthermia was studied as an adjunct to radiation therapy for the treatment of brain tumors.24 This phase I study involved 15 patients with grade III astrocytoma (n = 2) or GBM (n = 13). Five of the patients presented with primary tumors and underwent surgical debulking, while 10 patients presented with tumor recurrence following prior treatment. Following a craniectomy and the placement of thermocouple probes to measure the temperature in the region of interest, patients underwent weekly sonication sessions while receiving daily external beam radiotherapy. Hyperthermia was achieved using a 1.7 MHz curved transducer and a scanning mechanism. Over the course of 51 treatments, 41.9% of the thermocouple probes reached the target temperature of 42°C. The highly heterogeneous structure of the tumors was felt to result in non-uniform power deposition, thereby presenting a challenge to precise control over the procedure. Other limitations included impedance mismatches produced by aerated gelatin sponges and dural substitutes, attenuation by the temporalis muscle during the treatment of temporal lobe tumors, and the mitigating effect of bloodflow on heating in highly perfused tumors. Additionally, the need for a craniectomy as well as invasive temperature probes added surgical risk and morbidity to the procedure. However, with the recent advances in MRgFUS and MR thermometry, hyperthermia can now be achieved non-invasively and has become an even more attractive option for accomplishing radiosensitization.
Sonodynamic Therapy
An alternative approach to achieving cytotoxicity relies on sensitizing agents that absorb energy and produce reactive sonochemical species, ultimately resulting in cellular damage. Initially using light of particular wavelengths to activate these agents, photodynamic therapy (PDT) has been explored as a therapeutic option for brain tumors. However, light has poor penetration through most tissues (typically, a few millimeters), thereby requiring PDT to be applied intraoperatively or with interstitial fiberoptic sources.48,49 As a result, ultrasound is now being explored as a means of activating sensitizing agents and generating cytotoxicity—a process called sonodynamic therapy. FUS represents an attractive alternative to light-based activation, as it is not restricted by the same tissue penetration limitations.
The cytotoxic effects of combining ultrasound with sonosensitizers have been studied since 1989.50 In particular, pFUS has a minimal effect on temperature, and at low power is unlikely to damage cells on its own. Combined with a sonosensitizer, though, pFUS can produce significant cytotoxicity via a cavitation-based mechanism. The energy released by acoustic cavitation bubble collapse activates sonosensitizers and produces free radicals and singlet oxygen. These, in turn, trigger a chain reaction that culminates in lipid peroxidation and cytotoxicity on a cellular scale (for a review, see Chen et al51 and Rosenthal et al52).
A number of sonosensitizers have been investigated. Porphyrin derivatives represent one of the most widely studied groups of sensitizing agents; hematoporphyrin monomethyl ether (HMME), protoporphyrin IX (PpIX), and ATX-70 have been shown to have a sonodynamic effect in a variety of cancer types, including sarcoma,50 hepatocellular carcinoma,53 osteosarcoma,54 and endometrial cancer.55 Additionally, xanthenes, various chemotherapeutic agents (in particular, doxorubicin), second generation fluoroquinolones, and polyhydroxy fullerenes have all been shown to enhance the cytotoxic effects of ultrasound,51 although studies in brain tumor models are lacking.
With respect to brain tumors specifically, only a handful of sonosensitizers have been explored. Rose Bengal, for instance, has been shown to have a sonodynamic effect in rats implanted with C6 glioma cells26 but is limited by low levels of specific accumulation in tumor tissue. In particular, 5-aminolevulinic acid (5-Ala) has generated significant interest as a sensitizing agent. 5-Ala is metabolized to PpIX, an endogenous fluorescent bioproduct, as part of the heme biosynthesis pathway. In malignant glioma cells, but not in healthy brain cells, exposure to 5-Ala results in tumor-specific accumulation of PpIX as a result of alterations in enzymes and cell transporters involved in heme biosynthesis. These unique features have proven useful for the intraoperative discrimination of tumor and normal tissue in the operating room,56 as well as 5-Ala-based PDT.57
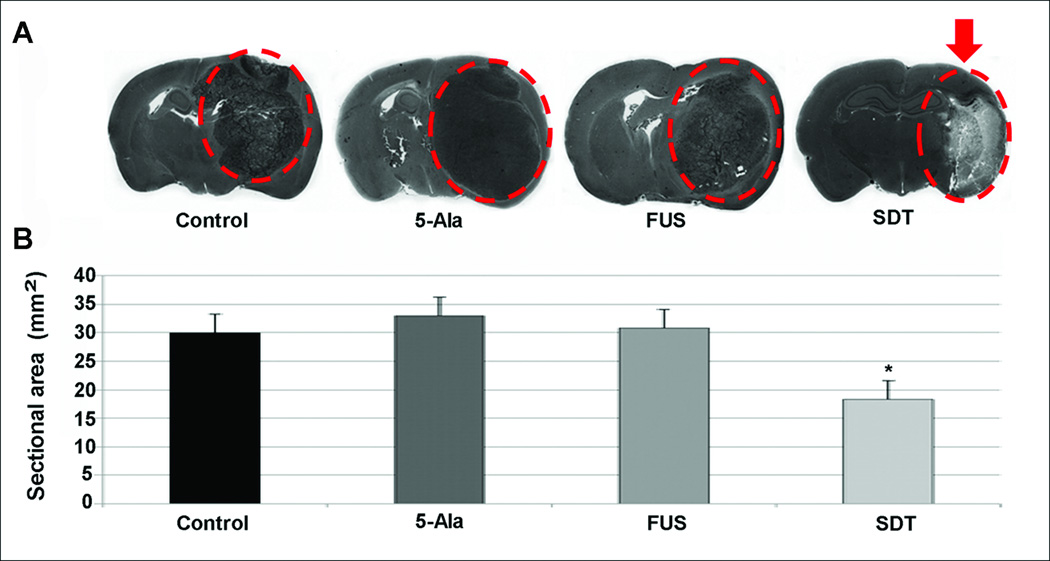
More recently, 5-Ala has also been investigated as a potential sonosensitizer. Ohmura et al27 first determined a safety threshold in the normal rat brain; when applying these sonication parameters to an orthotopic C6 glioma rat model 3 hours after the oral administration of 5-Ala, tumor volumes were smaller than in rats undergoing a sham operation, undergoing sonication alone, or receiving 5-Ala alone (Figure 2). The surrounding brain tissue was left undamaged. These results were later confirmed by Jeong et al25 using a lower ultrasound intensity. These early results are encouraging and warrant further research.
Figure 2.
Effect of SDT in a rat C6 intracerebral glioma model. A, coronal brain sections depicting selective destruction of the tumor (outlined by the dashed circle) in the group treated with SDT (arrow). B, tumor size in coronal sections for each treatment group. An asterisk indicates statistical significance (P < .05) when compared to rats undergoing a sham operation. 5-Ala, 5-aminolevulinic acid; FUS, focused ultrasound; SDT, sonodynamic therapy. Modified with permission from Ohmura T, Fukushima T, Shibaguchi H, et al. Sonodynamic therapy with 5-aminolevulinic acid and focused ultrasound for deep-seated intracranial glioma in rat. Anticancer Res. 2011;31(7):2527–2533.27
DRUG DELIVERY
While cytotoxicity is a natural goal of any oncologic therapy, MRgFUS is a versatile tool, with applications that extend beyond tumor ablation. A particularly well-studied area of research is the use of MRgFUS for enhancing drug delivery to brain tumors. Therapeutic agents that are systemically delivered must overcome a number of hurdles before reaching their targets within a brain cancer cell. These include the blood-brain barrier (BBB) and the brain tissue/tumor penetration barrier, each of which may be overcome by FUS.
Blood-Brain Barrier Disruption
The first (and most widely studied) obstacle to drug transport across the cerebral vasculature is the BBB, which is formed by specialized endothelial cells that are linked by tight junctions and lack fenestrations. Only small, lipophilic molecules less than 400 Daltons in size are capable of passively diffusing across the BBB. While this serves a protective function, it also prevents the vast majority of therapeutic agents from passively crossing the BBB, resulting in inadequate concentrations within the brain and increased systemic toxicity. Although the BBB is disrupted in many gliomas, it often remains intact at the periphery of the tumor, where invading tumor cells are interspersed with healthy brain cells. Delivering treatments to these invasive cells remains a significant challenge (for a review, see Woodworth et al58 and van Tellingen et al59).
Current strategies to manipulate or disrupt the BBB include the use of chimeric peptides,60 intra-arterial osmotic agents,61 alkylated alcohols,62 pro-inflammatory cytokines,63 and synthetic bradykinin analogs.64 However, some of these agents result in widespread and nonspecific BBB breakdown, allowing neural tissue to become exposed to toxic components of the blood. Others, such as chimeric peptides, are more specific but highly inefficient.
A promising alternative approach is to use MRgFUS to mechanically open the BBB in a targeted, localized region. The potential for acoustic energy to open the BBB was demonstrated as early as the 1950s, when trypan blue staining was identified in a sonicated region of the brain, without evidence of discrete damage.65 With the recent advances in MRgFUS technology, however, BBB disruption has become a prominent area of investigation. In order to achieve BBB disruption, pulsed exposures at low pressure amplitudes and relatively low frequencies compared to traditional therapeutic ultrasound treatments (~500 kHz vs 1 MHz) are provided immediately following the administration of intravenous ultrasound contrast agents (UCAs). These UCAs are typically lipid or albumin-encased gas microbubbles that are 1–5 microns in diameter and concentrate by the capillary walls, where they undergo oscillation in response to the varying pressure field of the ultrasound wave. The resulting stable (ie, non-inertial) cavitating bubbles exert stresses on the endothelial cell tight junctions, ultimately producing transient disruption of the BBB. Compromise of the BBB may endure up to 4–6 hours, depending on the molecular weight of the compound being transported (for a review, see Burgess and Hynynen66). Microbubbles enhance the effects of FUS, lowering the threshold for cavitation and allowing the acoustic energy to be delivered at non-destructive intensities to the surrounding tissues. Numerous preclinical studies have identified the ultrasound parameters that maximize BBB opening while minimizing tissue damage.67–70
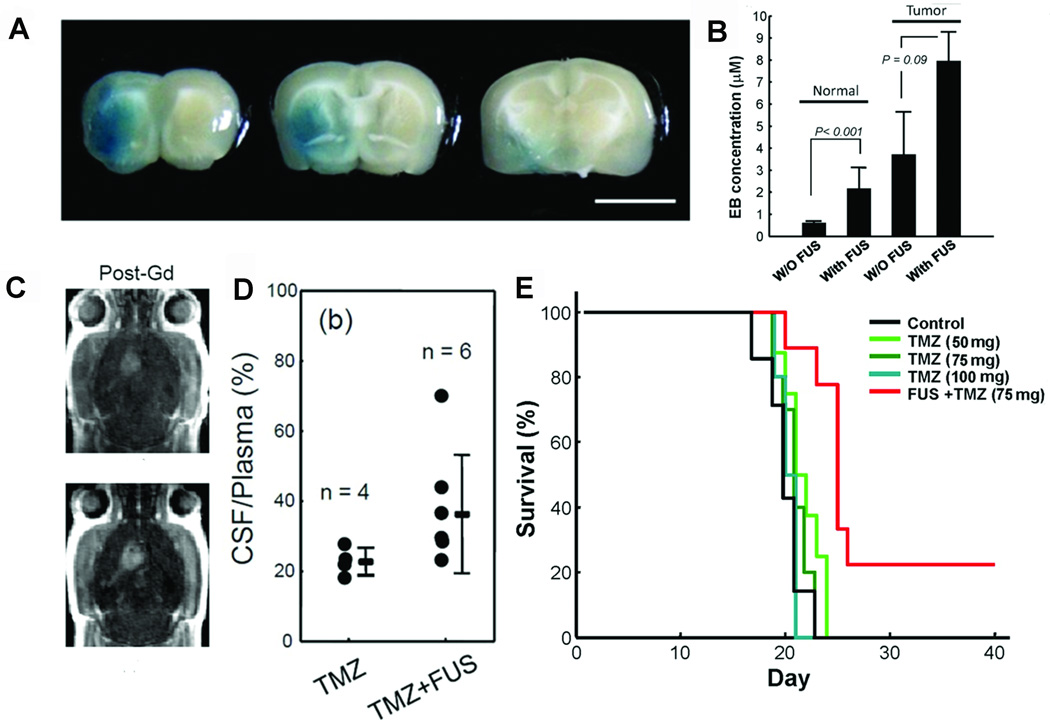
The ability to transiently disrupt the BBB without causing tissue damage has the potential to dramatically alter the landscape of drug delivery to the brain. MRgFUS, in combination with microbubbles, has already been used in preclinical models to enhance the delivery of a number of agents that are typically too large to cross the BBB. This approach was first successfully demonstrated using liposome-encapsulated doxorubicin,71–73 with subsequent studies examining intravenous methotrexate,74 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU),75 temozolomide,76 and the monoclonal antibody traztuzumab (Figure 3).77,78 Recently, repeated opening of the BBB was achieved in the 9L rat glioma model using MRgFUS. Three weekly sessions of ultrasound-induced BBB disruption increased the intracerebral concentration of intravenously delivered liposomal doxorubicin, resulting in clinically relevant concentrations and significantly longer median survival (P < .001).79,80 The safety of these treatments was confirmed by repeatedly disrupting the BBB in the central visual field targets or basal ganglia of non-human primates. BBB opening in visual field targets was successful in 163 of 185 targeted locations, while in the basal ganglia the BBB was successfully opened in 24 of 25 FUS treatments. The animals did not demonstrate any long-term visual or motor deficits.81,82
Figure 3.
Ultrasound-mediated opening of the BBB. A, coronal brain sections depicting extravasation of Evans blue dye (EBD) following FUS-mediated opening of the BBB (scale = 5 mm). B, quantification of EBD concentration with or without FUS in normal rats (sonication produced a 3.8-fold increase, P < .001) and in rats with tumors (sonication produced a 2.1-fold increase, P = .09). C, axial, contrast-enhanced T1-weighted magnetic resonance imaging sequences demonstrating increased contrast-enhancement following sonication of a brain tumor in a rat (lower) relative to a tumor that did not undergo sonication (upper). D, CSF-to-plasma ratio of the TMZ concentration in animals treated with TMZ only or combined TMZ with FUS-BBB opening (TMZ + FUS). E, Kaplan-Meier plot demonstrating improved survival of animals in the TMZ + FUS group compared to control animals or those receiving TMZ of various doses alone. BBB, blood-brain barrier; EBD, Evans blue dye; FUS, focused ultrasound; TMZ, temozolomide. Modified with permission from Wei KC, Chu PC, Wang HY, et al. Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PLoS One. 2013;8(3):e58995.76
The abundance of preclinical work demonstrating the safety and efficacy of BBB disruption using FUS has culminated in an ongoing phase I trial (NCT02343991) of MRgFUS with intravenous microbubbles in brain tumor patients who are being treated with doxorubicin. The first patient to be enrolled in the trial was recently treated, and the BBB was successfully opened. Confirmation was obtained via contrast enhancement on the post-sonication MRI, and the patient was subsequently taken to the operating room for surgical resection. Using intraoperative neuronavigation, the surgeon was able to select areas in which the BBB was or was not opened, and analysis of the drug concentrations in the corresponding regions is currently ongoing. Additional patients are being recruited for the study, and once the safety of the technique is established in humans, future studies will further evaluate its efficacy.
Ultrasound-Assisted Local Delivery
Once an agent has overcome the BBB, another critical barrier to achieving the desired therapeutic effect is the tissue/tumor penetration barrier.58 This is formed by the anisotropic, electrostatically charged extracellular space (ECS) and glialymphatic system (GLS) that comprise the space between brain cells.83 The extracellular matrix (ECM), in particular, accounts for 10%–20% of the total brain volume and forms a dense network that limits the dispersion of therapeutic agents, even when they are delivered locally via surgically implanted drug-loaded interstitial wafers or catheter-based convection-enhanced delivery (CED).84 The limited distribution of drugs and other therapeutics to areas of the brain that have been invaded by tumor cells is a major limitation to current treatment. One recent approach being explored involves the engineering of “brain-penetrating” nanoparticles with a dense poly(ethylene glycol) coating that prevents non-specific binding to components of the brain ECM.85–87 Ultrasound, however, may produce tissue effects that complement these innovative nanoparticle formulations.
Preclinical work has demonstrated that pFUS can safely produce mechanical effects that alter tissue architecture and improve permeability in tumors outside of the CNS, with significant improvement on the distribution of locally administered agents. In one study, pFUS exposures not only improved the distribution of fluorescent polystyrene nanoparticles in squamous cell carcinoma xenografts, but also enhanced the efficacy of tumor necrosis factor alpha (TNFα) plasmid injections. Electron microscopy revealed gaps, sometimes microns in size, between the tumor cells exposed to pFUS.88
Whether similar effects can be produced in brain tissue is currently under investigation. Liu et al89 performed a range of in vitro and in vivo studies with pulses of unfocused ultrasound. Using excised porcine brain tissue and orthotopic 9L tumors, they demonstrated that ultrasound increases brain tissue and tumor permeability to variably sized3H-labeled molecules (water, mannitol, inulin, and dextran) increased by 2.3- to 8.8-fold following sonication, with smaller molecules demonstrating larger increases in permeability. Additional in vivo work in a primate model showed that 1 MHz unfocused pulsed ultrasound (applied through a craniotomy) enhanced the distribution of liposomes containing an MR contrast agent following CED.
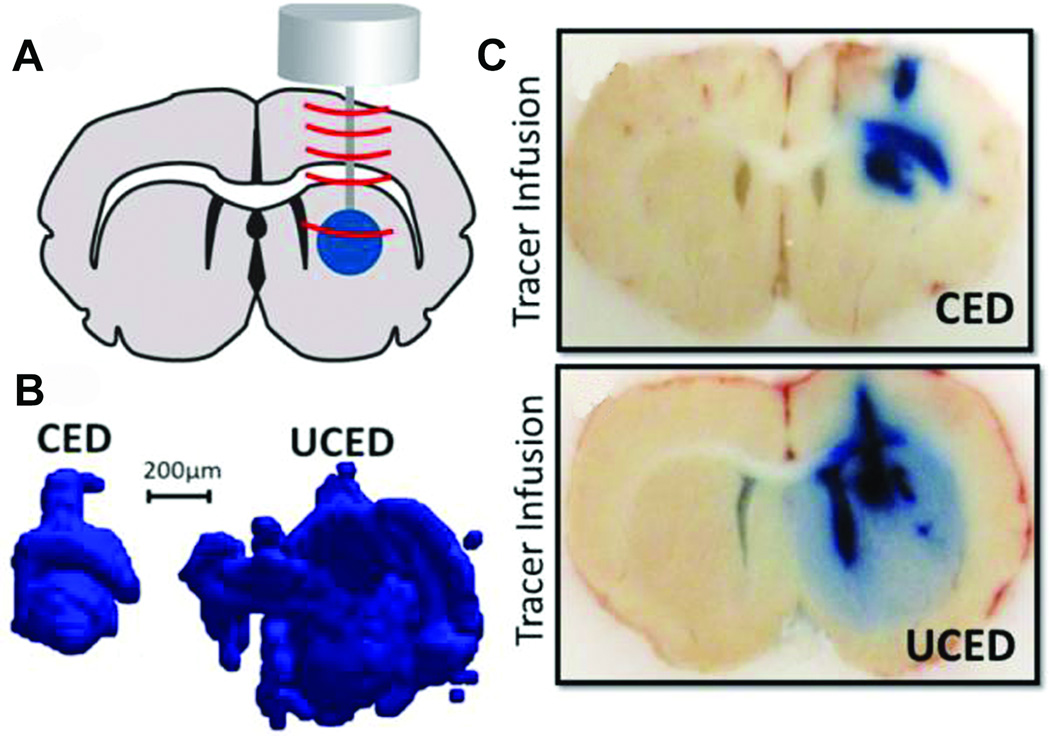
Lewis et al90 expanded on this work, using a transducer cannula assembly to apply low-intensity, continuous, unfocused ultrasound during CED (Figure 4). Sonication increased the volumetric distribution of Evans blue dye (EBD) injected into the caudate nucleus of rats by a factor of 2.24 to 3.25, although, for unclear reasons, adding microbubbles to the infusate reduced the distribution of dye. The authors hypothesized that the microbubbles may have been confined to the tip of the CED catheter; alternatively, they may have attenuated the acoustic energy. A subsequent study used the time-reversal acoustics method to apply focused ultrasound with a custom-designed “smart needle” that incorporated an infusion catheter with a piezo-electric ultrasound transducer.91 Using this technique, the distribution of EBD increased by a factor of 7.2 in brain-mimicking agarose gels, and by 75% in the caudate nucleus of anesthetized rodents. In this case, microbubble administration slightly improved the distribution of dye. However, further studies are needed to determine the true effects of ultrasound on brain cytoarchitecture in an effort to better understand this phenomenon.
Figure 4.
Ultrasound-assisted convection-enhanced delivery (UCED) to the rodent brain. A, a plane wave transducer with a cannula through-hole is mounted on top of the rodent brain through a small craniotomy window in the skull. B, 3-dimensional reconstruction of the distribution of infused EBD with and without ultrasound exposure. C, brain slices in the cannula path showing EBD distribution with (lower) and without (upper) ultrasound exposure. CED, convection-enhanced delivery; EBD, Evans blue dye; UCED, ultrasound-assisted convection-enhanced delivery. Reproduced from Lewis GK Jr., Guarino S, Ghandi G, et al. Time-reversal techniques in ultrasound-assisted convection-enhanced drug delivery to the brain: technology development and in vivo evaluation. Proc Meet Acoust. 2011;11:20005–20031,107 with the permission of the Acoustical Society of America.
IMMUNOMODULATION
The development of immunotherapy, which involves priming the host’s immune system to recognize a tumor as foreign material, has been an expanding area of GBM research. FUS may indirectly contribute to this process, as evidence has shown that tumor ablation produces cellular debris that contains tumor-specific antigens (TSAs)92 and may also enhance tumor-specific T cell activity.93,94 In mice with subcutaneous C1300 neuroblastoma tumors, FUS-induced ablation reduced tumor growth in animals that were re-challenged, presumably due to the immunomodulatory effects of the initial ablation.95 The debris produced by FUS has even been used to produce a vaccine to activate the immune system and induce a tumor-specific response in a mouse model of hepatocellular carcinoma.96 A number of mechanisms have been proposed. On the one hand, thermal ablation may relieve the immunosuppression caused by the tumor and may upregulate heat shock proteins (HSPs) such as HSP70, an intracellular molecular chaperone that can modulate the immunogenicity of tumors by presenting TSAs to antigen presenting cells (APCs). Indeed, hyperthermia has been implicated as a modulator of gene expression in T-lymphocytes.97
However, mounting evidence suggests that it is not the thermal effects at the center of the focal beam, but rather effects that occur at the border of the ablative lesion that play the dominant role. In these regions, intensities are insufficient to generate cytotoxic heat, but still high enough to generate destructive mechanical effects. In an in vitro study, mechanical exposures resulted in the release of HSP60, an endogenous danger signal, by tumor cells, which in turn activated APCs, resulting in increased secretion of TNF-α and interleukin-12 by macrophages and dendritic cells, respectively.98 This work was followed by in vivo studies using mice with MC-38 colon adenocarcinoma,99 H22 hepatocellular carcinoma,96,100 and RM-9 prostate cancer tumors.101 In these studies, pFUS exposures generating primarily mechanical effects resulted in increased infiltration of dendritic cells into the primary tumor, elevated cytotoxic T-lymphocyte activity, increased production of tumor-specific interferon gamma, down-regulation of signal transducer and activator of transcription 3 (STAT3), and slower tumor growth upon re-challenge.
Clinical studies have confirmed an enhanced immune response following FUS treatments in patients. Several groups have documented the preservation of some tumor antigens in the tumor debris following FUS treatment102 as well as increased levels of natural killer cells and CD4+ T-lymphocytes,103 improved CD4+ to CD8+ ratios,104,105 and decreased levels of immunosuppressive cytokines such as VEGF and transforming growth factor-(TGF) β1.106 Despite mounting preclinical and clinical evidence, studies involving tumors of the central nervous system are still lacking.
CONCLUSION
MRgFUS offers exciting options for the noninvasive treatment of numerous neurological disorders. Within the field of neuro-oncology, the ablative applications of MRgFUS are the closest to being implemented clinically, with several clinical trials already exploring the technology’s safety and feasibility in patients. However, thermal ablation is only one of several potential applications of FUS. Other applications currently being explored include SDT, radiosensitization, drug delivery, and immunomodulation. Most of this work has been performed in other organ systems, and further studies of ultrasound’s effects in the unique microenvironment of the brain are necessary if we are to fully take advantage of this innovative technology. Additional applications in the future could also include the use of ultrasound to generate low-level, nondestructive heat for deploying therapeutic agents from thermosensitive liposomes or activating heat shock promoters for spatiotemporal control of transgene expression. Pre-clinical reports on these targeted applications have generated much enthusiasm, and the feasibility for clinical translation is supported by the current state of the technology. As the technology is refined even further, we can expect to see FUS take its place as a valuable adjuvant therapy in the fight against brain cancer.
Acknowledgments
This work was supported in part by the National Institutes of Health (K12NS080223 [GFW], K25EB018370 [AJK], K08NS09043 [GFW]), an American Medical Association Foundation Seed Grant (DSH), a grant from the Focused Ultrasound Foundation External Awards Program High-Risk Track (VF), a Dean’s Challenge Award to Accelerate Innovation and Discovery in Medicine (VF, JAW, and GFW), a Department of Defense Congressionally Directed Medical Research Programs Lung Cancer Research Program IDEA Award (W81XWH-14-1-0324) (JAW), an Institutional Research Grant (IRG-97-153-10) from the American Cancer Society (AJK and GFW), a Passano Foundation Physician Scientist Award (GFW), an Elsa U. Pardee Foundation Research Grant (AJK and JAW), a Pharmaceutical Research and Manufacturers of America Foundation Research Starter Grant in Pharmaceutics (AJK), an American Association of Pharmaceutical Scientists Foundation New Investigator Grant Award (AJK), and the Department of Defense PTSD/TBI Clinical Consortium (HME). Dr Eisenberg is an unpaid consultant for InSightec, Ltd, and is the principal investigator of clinical trials sponsored by InSightec and the Focused Ultrasound Foundation (NCT02289560 and NCT02263885). Dr Eisenberg and Dr Woodworth are also independent neuro-trauma consultants to the National Football League.
Footnotes
Disclosures: Drs Hersh, Kim, Winkles, Woodworth, and Frenkel have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1.Lynn JG, Putnam TJ. Histology of cerebral lesions produced by focused ultrasound. Am J Pathol. 1944;20(3):637–649. [PMC free article] [PubMed] [Google Scholar]
- 2.Fry FJ. Precision high intensity focusing ultrasonic machines for surgery. Am J Phys Med. 1958;37(3):152–156. [PubMed] [Google Scholar]
- 3.Fry WJ, Mosberg WH, Jr, Barnard JW, Fry FJ. Production of focal destructive lesions in the central nervous system with ultrasound. J Neurosurg. 1954;11(5):471–478. doi: 10.3171/jns.1954.11.5.0471. [DOI] [PubMed] [Google Scholar]
- 4.Hynynen K, Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med Biol. 1998;24(2):275–283. doi: 10.1016/s0301-5629(97)00269-x. [DOI] [PubMed] [Google Scholar]
- 5.Cline HE, Hynynen K, Hardy CJ, Watkins RD, Schenck JF, Jolesz FA. MR temperature mapping of focused ultrasound surgery. Magn Reson Med. 1994;31(6):628–636. doi: 10.1002/mrm.1910310608. [DOI] [PubMed] [Google Scholar]
- 6.Medel R, Monteith SJ, Elias WJ, et al. Magnetic resonance-guided focused ultrasound surgery Part 2: A review of current and future applications. Neurosurgery. 2012;71(4):755–763. doi: 10.1227/NEU.0b013e3182672ac9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sloan AE, Ahluwalia MS, Valerio-Pascua J, et al. Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma: clinical article. J Neurosurg. 2013;118(6):1202–1219. doi: 10.3171/2013.1.JNS1291. [DOI] [PubMed] [Google Scholar]
- 8.Mohammadi AM, Hawasli AH, Rodriguez A, et al. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: a multicenter study. Cancer Med. 2014;3(4):971–979. doi: 10.1002/cam4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haar GT, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia. 2007;23(2):89–104. doi: 10.1080/02656730601186138. [DOI] [PubMed] [Google Scholar]
- 10.Ghanouni P, Pauly KB, Elias WJ, et al. Transcranial MRI-guided focused ultrasound: a review of the technologic and neurologic applications. AJR Am J Roentgenol. 2015;205(1):150–159. doi: 10.2214/AJR.14.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlesinger D, Benedict S, Diederich C, Gedroyc W, Klibanov A, Larner J. MR-guided focused ultrasound surgery, present and future. Med Phys. 2013;40(8):080901. doi: 10.1118/1.4811136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tempany CM, McDannold NJ, Hynynen K, Jolesz FA. Focused ultrasound surgery in oncology: overview and principles. Radiology. 2011;259(1):39–56. doi: 10.1148/radiol.11100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5(4):321–327. doi: 10.1038/nrc1591. [DOI] [PubMed] [Google Scholar]
- 14.O'Neill BE, Vo H, Angstadt M, Li KP, Quinn T, Frenkel V. Pulsed high intensity focused ultrasound mediated nanoparticle delivery: mechanisms and efficacy in murine muscle. Ultrasound Med Biol. 2009;35(3):416–424. doi: 10.1016/j.ultrasmedbio.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frenkel V, Etherington A, Greene M, et al. Delivery of liposomal doxorubicin (Doxil) in a breast cancer tumor model: investigation of potential enhancement by pulsed-high intensity focused ultrasound exposure. Acad Radiol. 2006;13(4):469–479. doi: 10.1016/j.acra.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Krasovitski B, Frenkel V, Shoham S, Kimmel E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc Natl Acad Sci U S A. 2011;108(8):3258–3263. doi: 10.1073/pnas.1015771108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hancock HA, Smith LH, Cuesta J, et al. Investigations into pulsed high-intensity focused ultrasound-enhanced delivery: preliminary evidence for a novel mechanism. Ultrasound Med Biol. 2009;35(10):1722–1736. doi: 10.1016/j.ultrasmedbio.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frenkel V, Kimmel E, Iger Y. Ultrasound-facilitated transport of silver chloride (AgCl) particles in fish skin. J Control Release. 2000;68(2):251–261. doi: 10.1016/s0168-3659(00)00264-9. [DOI] [PubMed] [Google Scholar]
- 19.Frenkel V, Gurka R, Liberzon A, Shavit U, Kimmel E. Preliminary investigations of ultrasound induced acoustic streaming using particle image velocimetry. Ultrasonics. 2001;39(3):153–156. doi: 10.1016/s0041-624x(00)00064-0. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy JE, Wu F, ter Haar GR, et al. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics. 2004;42(1–9):931–935. doi: 10.1016/j.ultras.2004.01.089. [DOI] [PubMed] [Google Scholar]
- 21.Thuroff S, Chaussy C, Vallancien G, et al. High-intensity focused ultrasound and localized prostate cancer: efficacy results from the European multicentric study. J Endourol. 2003;17(8):673–677. doi: 10.1089/089277903322518699. [DOI] [PubMed] [Google Scholar]
- 22.Ram Z, Cohen ZR, Harnof S, et al. Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumor therapy. Neurosurgery. 2006;59(5):949–955. doi: 10.1227/01.NEU.0000254439.02736.D8. discussion 955-946. [DOI] [PubMed] [Google Scholar]
- 23.Liberman B, Gianfelice D, Inbar Y, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann Surg Oncol. 2009;16(1):140–146. doi: 10.1245/s10434-008-0011-2. [DOI] [PubMed] [Google Scholar]
- 24.Guthkelch AN, Carter LP, Cassady JR, et al. Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: results of a phase I trial. J Neurooncol. 1991;10(3):271–284. doi: 10.1007/BF00177540. [DOI] [PubMed] [Google Scholar]
- 25.Jeong EJ, Seo SJ, Ahn YJ, Choi KH, Kim KH, Kim JK. Sonodynamically induced antitumor effects of 5-aminolevulinic acid and fractionated ultrasound irradiation in an orthotopic rat glioma model. Ultrasound Med Biol. 2012;38(12):2143–2150. doi: 10.1016/j.ultrasmedbio.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 26.Nonaka M, Yamamoto M, Yoshino S, Umemura S, Sasaki K, Fukushima T. Sonodynamic therapy consisting of focused ultrasound and a photosensitizer causes a selective antitumor effect in a rat intracranial glioma model. Anticancer Res. 2009;29(3):943–950. [PubMed] [Google Scholar]
- 27.Ohmura T, Fukushima T, Shibaguchi H, et al. Sonodynamic therapy with 5-aminolevulinic acid and focused ultrasound for deep-seated intracranial glioma in rat. Anticancer Res. 2011;31(7):2527–2533. [PubMed] [Google Scholar]
- 28.Cline HE, Hynynen K, Watkins RD, et al. Focused US system for MR imaging-guided tumor ablation. Radiology. 1995;194(3):731–737. doi: 10.1148/radiology.194.3.7862971. [DOI] [PubMed] [Google Scholar]
- 29.Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19(3):267–294. doi: 10.1080/0265673031000119006. [DOI] [PubMed] [Google Scholar]
- 30.Stewart EA, Gedroyc WM, Tempany CM, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol. 2003;189(1):48–54. doi: 10.1067/mob.2003.345. [DOI] [PubMed] [Google Scholar]
- 31.Thuroff S, Chaussy C, Vallancien G, et al. High-intensity focused ultrasound and localized prostate cancer: efficacy results from the European multicentric study. J Endourol. 2003;17(8):673–677. doi: 10.1089/089277903322518699. [DOI] [PubMed] [Google Scholar]
- 32.Pron G. Magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) treatment of symptomatic uterine fibroids: an evidence-based analysis. Ont Health Technol Assess Ser. 2015;15(4):1–86. [PMC free article] [PubMed] [Google Scholar]
- 33.Chaussy C, Thuroff S. High-intensity focused ultrasound in the management of prostate cancer. Expert Rev Med Devices. 2010;7(2):209–217. doi: 10.1586/erd.09.66. [DOI] [PubMed] [Google Scholar]
- 34.Crouzet S, Murat FJ, Pasticier G, Cassier P, Chapelon JY, Gelet A. High intensity focused ultrasound (HIFU) for prostate cancer: current clinical status, outcomes and future perspectives. Int J Hyperthermia. 2010;26(8):796–803. doi: 10.3109/02656736.2010.498803. [DOI] [PubMed] [Google Scholar]
- 35.McDannold N, Clement GT, Black P, Jolesz F, Hynynen K. Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery. 2010;66(2):323–332. doi: 10.1227/01.NEU.0000360379.95800.2F. discussion 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. 10. [DOI] [PubMed] [Google Scholar]
- 37.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 38.Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):45–68. doi: 10.1007/s11060-009-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyu X, Zheng D, Zhang H, et al. Hyperthermia improves immune function and radiotherapy efficacy in patients with post-operative recurrent gastric cancer. Hepatogastroenterology. 2014;61(136):2428–2433. [PubMed] [Google Scholar]
- 40.Westermann A, Mella O, Van Der Zee J, et al. Long-term survival data of triple modality treatment of stage IIB-III-IVA cervical cancer with the combination of radiotherapy, chemotherapy and hyperthermia - an update. Int J Hyperthermia. 2012;28(6):549–553. doi: 10.3109/02656736.2012.673047. [DOI] [PubMed] [Google Scholar]
- 41.Varma S, Myerson R, Moros E, Taylor M, Straube W, Zoberi I. Simultaneous radiotherapy and superficial hyperthermia for high-risk breast carcinoma: a randomised comparison of treatment sequelae in heated versus non-heated sectors of the chest wall hyperthermia. Int J Hyperthermia. 2012;28(7):583–590. doi: 10.3109/02656736.2012.705216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sneed PK, Stauffer PR, McDermott MW, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/− hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1998;40(2):287–295. doi: 10.1016/s0360-3016(97)00731-1. [DOI] [PubMed] [Google Scholar]
- 43.Krawczyk PM, Eppink B, Essers J, et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci U S A. 2011;108(24):9851–9856. doi: 10.1073/pnas.1101053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Genet SC, Fujii Y, Maeda J, et al. Hyperthermia inhibits homologous recombination repair and sensitizes cells to ionizing radiation in a time- and temperature-dependent manner. J Cell Physiol. 2013;228(7):1473–1481. doi: 10.1002/jcp.24302. [DOI] [PubMed] [Google Scholar]
- 45.Westra A, Dewey WC. Variation in sensitivity to heat shock during the cell-cycle of Chinese hamster cells in vitro. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;19(5):467–477. doi: 10.1080/09553007114550601. [DOI] [PubMed] [Google Scholar]
- 46.Man J, Shoemake JD, Ma T, et al. Hyperthermia sensitizes glioma stem-like cells to radiation by inhibiting AKT signaling. Cancer Res. 2015;75(8):1760–1769. doi: 10.1158/0008-5472.CAN-14-3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawasli AH, Kim AH, Dunn GP, Tran DD, Leuthardt EC. Stereotactic laser ablation of high-grade gliomas. Neurosurg Focus. 2014;37(6):E1. doi: 10.3171/2014.9.FOCUS14471. [DOI] [PubMed] [Google Scholar]
- 48.Powers SK, Brown JT. Light dosimetry in brain tissue: an in vivo model applicable to photodynamic therapy. Lasers Surg Med. 1986;6(3):318–322. doi: 10.1002/lsm.1900060305. [DOI] [PubMed] [Google Scholar]
- 49.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 50.Yumita N, Nishigaki R, Umemura K, Umemura S. Hematoporphyrin as a sensitizer of cell-damaging effect of ultrasound. Jpn J Cancer Res. 1989;80(3):219–222. doi: 10.1111/j.1349-7006.1989.tb02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H, Zhou X, Gao Y, Zheng B, Tang F, Huang J. Recent progress in development of new sonosensitizers for sonodynamic cancer therapy. Drug Discov Today. 2014;19(4):502–509. doi: 10.1016/j.drudis.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 52.Rosenthal I, Sostaric JZ, Riesz P. Sonodynamic therapy - a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem. 2004;11(6):349–363. doi: 10.1016/j.ultsonch.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Yumita N, Umemura S. Sonodynamic therapy with photofrin II on AH130 solid tumor. Pharmacokinetics, tissue distribution and sonodynamic antitumoral efficacy of photofrin II. Cancer Chemother Pharmacol. 2003;51(2):174–178. doi: 10.1007/s00280-002-0523-6. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Zhou Q, Hu Z, et al. 5-Aminolevulinic acid-based sonodynamic therapy induces the apoptosis of osteosarcoma in mice. PLoS One. 2015;10(7):e0132074. doi: 10.1371/journal.pone.0132074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun H, Ge W, Gao X, et al. Apoptosis-promoting effects of hematoporphyrin monomethyl ether-sonodynamic therapy (HMME-SDT) on endometrial cancer. PLoS One. 2015;10(9):e0137980. doi: 10.1371/journal.pone.0137980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 57.Stummer W, Beck T, Beyer W, et al. Long-sustaining response in a patient with non-resectable, distant recurrence of glioblastoma multiforme treated by interstitial photodynamic therapy using 5-ALA: case report. J Neurooncol. 2008;87(1):103–109. doi: 10.1007/s11060-007-9497-x. [DOI] [PubMed] [Google Scholar]
- 58.Woodworth GF, Dunn GP, Nance EA, Hanes J, Brem H. Emerging insights into barriers to effective brain tumor therapeutics. Front Oncol. 2014;4:126. doi: 10.3389/fonc.2014.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 60.Shin SU, Friden P, Moran M, et al. Transferrin-antibody fusion proteins are effective in brain targeting. Proc Natl Acad Sci U S A. 1995;92(7):2820–2824. doi: 10.1073/pnas.92.7.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neuwelt EA, Howieson J, Frenkel EP, et al. Therapeutic efficacy of multiagent chemotherapy with drug delivery enhancement by blood-brain barrier modification in glioblastoma. Neurosurgery. 1986;19(4):573–582. doi: 10.1227/00006123-198610000-00011. [DOI] [PubMed] [Google Scholar]
- 62.Erdlenbruch B, Alipour M, Fricker G, et al. Alkylglycerol opening of the blood-brain barrier to small and large fluorescence markers in normal and C6 glioma-bearing rats and isolated rat brain capillaries. Br J Pharmacol. 2003;140(7):1201–1210. doi: 10.1038/sj.bjp.0705554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huppert J, Closhen D, Croxford A, et al. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. Faseb J. 2010;24(4):1023–1034. doi: 10.1096/fj.09-141978. [DOI] [PubMed] [Google Scholar]
- 64.Matsukado K, Inamura T, Nakano S, Fukui M, Bartus RT, Black KL. Enhanced tumor uptake of carboplatin and survival in glioma-bearing rats by intracarotid infusion of bradykinin analog, RMP-7. Neurosurgery. 1996;39(1):125–133. doi: 10.1097/00006123-199607000-00025. discussion 133-124. [DOI] [PubMed] [Google Scholar]
- 65.Ballantine HT, Jr, Bell E, Manlapaz J. Progress and problems in the neurological applications of focused ultrasound. J Neurosurg. 1960;17:858–876. doi: 10.3171/jns.1960.17.5.0858. [DOI] [PubMed] [Google Scholar]
- 66.Burgess A, Hynynen K. Drug delivery across the blood-brain barrier using focused ultrasound. Expert Opin Drug Deliv. 2014;11(5):711–721. doi: 10.1517/17425247.2014.897693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang FY, Fu WM, Yang RS, Liou HC, Kang KH, Lin WL. Quantitative evaluation of focused ultrasound with a contrast agent on blood-brain barrier disruption. Ultrasound Med Biol. 2007;33(9):1421–1427. doi: 10.1016/j.ultrasmedbio.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 68.McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood-brain barrier with focused ultrasound: association with cavitation activity. Phys Med Biol. 2006;51(4):793–807. doi: 10.1088/0031-9155/51/4/003. [DOI] [PubMed] [Google Scholar]
- 69.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220(3):640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 70.Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24(1):12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 71.Kovacs Z, Werner B, Rassi A, Sass JO, Martin-Fiori E, Bernasconi M. Prolonged survival upon ultrasound-enhanced doxorubicin delivery in two syngenic glioblastoma mouse models. J Control Release. 2014;187:74–82. doi: 10.1016/j.jconrel.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 72.Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121(4):901–907. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- 73.Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol. 2012;38(10):1716–1725. doi: 10.1016/j.ultrasmedbio.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mei J, Cheng Y, Song Y, et al. Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging-guided focused ultrasound. J Ultrasound Med. 2009;28(7):871–880. doi: 10.7863/jum.2009.28.7.871. [DOI] [PubMed] [Google Scholar]
- 75.Liu HL, Hua MY, Yang HW, et al. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc Natl Acad Sci U S A. 2010;107(34):15205–15210. doi: 10.1073/pnas.1003388107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei KC, Chu PC, Wang HY, et al. Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PLoS One. 2013;8(3):e58995. doi: 10.1371/journal.pone.0058995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park EJ, Zhang YZ, Vykhodtseva N, McDannold N. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J Control Release. 2012;163(3):277–284. doi: 10.1016/j.jconrel.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A. 2006;103(31):11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aryal M, Vykhodtseva N, Zhang YZ, McDannold N. Multiple sessions of liposomal doxorubicin delivery via focused ultrasound mediated blood-brain barrier disruption: a safety study. J Control Release. 2015;204:60–69. doi: 10.1016/j.jconrel.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aryal M, Vykhodtseva N, Zhang YZ, Park J, McDannold N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J Control Release. 2013;169(1–2):103–111. doi: 10.1016/j.jconrel.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012;72(14):3652–3663. doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Downs ME, Buch A, Sierra C, et al. Long-term safety of repeated blood-brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task. PLoS One. 2015;10(5):e0125911. doi: 10.1371/journal.pone.0125911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev. 2008;88(4):1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nance EA, Woodworth GF, Sailor KA, et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med. 2012;4(149):149ra119. doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schneider CS, Perez JG, Cheng E, et al. Minimizing the non-specific binding of nanoparticles to the brain enables active targeting of Fn14-positive glioblastoma cells. Biomaterials. 2015;42:42–51. doi: 10.1016/j.biomaterials.2014.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou J, Patel TR, Sirianni RW, et al. Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proc Natl Acad Sci U S A. 2013;110(29):11751–11756. doi: 10.1073/pnas.1304504110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ziadloo A, Xie J, Frenkel V. Pulsed focused ultrasound exposures enhance locally administered gene therapy in a murine solid tumor model. J Acoust Soc Am. 2013;133(3):1827–1834. doi: 10.1121/1.4789390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Y, Paliwal S, Bankiewicz KS, et al. Ultrasound-enhanced drug transport and distribution in the brain. AAPS PharmSciTech. 2010;11(3):1005–1017. doi: 10.1208/s12249-010-9458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lewis GK, Jr, Schulz ZR, Pannullo SC, Southard TL, Olbricht WL. Ultrasound-assisted convection-enhanced delivery to the brain in vivo with a novel transducer cannula assembly: laboratory investigation. J Neurosurg. 2012;117(6):1128–1140. doi: 10.3171/2012.7.JNS11144. [DOI] [PubMed] [Google Scholar]
- 91.Olbricht W, Sistla M, Ghandi G, Lewis G, Jr, Sarvazyan A. Time-reversal acoustics and ultrasound-assisted convection-enhanced drug delivery to the brain. J Acoust Soc Am. 2013;134(2):1569–1575. doi: 10.1121/1.4812879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.den Brok MH, Sutmuller RP, van der Voort R, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64(11):4024–4029. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 93.Sanchez-Ortiz RF, Tannir N, Ahrar K, Wood CG. Spontaneous regression of pulmonary metastases from renal cell carcinoma after radio frequency ablation of primary tumor: an in situ tumor vaccine? J Urol. 2003;170(1):178–179. doi: 10.1097/01.ju.0000070823.38336.7b. [DOI] [PubMed] [Google Scholar]
- 94.Wissniowski TT, Hansler J, Neureiter D, et al. Activation of tumor-specific T lymphocytes by radio-frequency ablation of the VX2 hepatoma in rabbits. Cancer Res. 2003;63(19):6496–6500. [PubMed] [Google Scholar]
- 95.Yang R, Reilly CR, Rescorla FJ, et al. Effects of high-intensity focused ultrasound in the treatment of experimental neuroblastoma. J Pediatr Surg. 1992;27(2):246–250. doi: 10.1016/0022-3468(92)90321-w. discussion 250-241. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y, Deng J, Feng J, Wu F. Enhancement of antitumor vaccine in ablated hepatocellular carcinoma by high-intensity focused ultrasound. World J Gastroenterol. 2010;16(28):3584–3591. doi: 10.3748/wjg.v16.i28.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cippitelli M, Fionda C, Di Bona D, Piccoli M, Frati L, Santoni A. Hyperthermia enhances CD95-ligand gene expression in T lymphocytes. J Immunol. 2005;174(1):223–232. doi: 10.4049/jimmunol.174.1.223. [DOI] [PubMed] [Google Scholar]
- 98.Hu Z, Yang XY, Liu Y, et al. Release of endogenous danger signals from HIFU-treated tumor cells and their stimulatory effects on APCs. Biochem Biophys Res Commun. 2005;335(1):124–131. doi: 10.1016/j.bbrc.2005.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu Z, Yang XY, Liu Y, et al. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J Transl Med. 2007;5:34. doi: 10.1186/1479-5876-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xia JZ, Xie FL, Ran LF, Xie XP, Fan YM, Wu F. High-intensity focused ultrasound tumor ablation activates autologous tumor-specific cytotoxic T lymphocytes. Ultrasound Med Biol. 2012;38(8):1363–1371. doi: 10.1016/j.ultrasmedbio.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 101.Huang X, Yuan F, Liang M, et al. M-HIFU inhibits tumor growth, suppresses STAT3 activity and enhances tumor specific immunity in a transplant tumor model of prostate cancer. PLoS One. 2012;7(7):e41632. doi: 10.1371/journal.pone.0041632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu F, Wang ZB, Cao YD, et al. Expression of tumor antigens and heat-shock protein 70 in breast cancer cells after high-intensity focused ultrasound ablation. Ann Surg Oncol. 2007;14(3):1237–1242. doi: 10.1245/s10434-006-9275-6. [DOI] [PubMed] [Google Scholar]
- 103.Lu P, Zhu XQ, Xu ZL, Zhou Q, Zhang J, Wu F. Increased infiltration of activated tumor-infiltrating lymphocytes after high intensity focused ultrasound ablation of human breast cancer. Surgery. 2009;145(3):286–293. doi: 10.1016/j.surg.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 104.Rosberger DF, Coleman DJ, Silverman R, Woods S, Rondeau M, Cunningham-Rundles S. Immunomodulation in choroidal melanoma: reversal of inverted CD4/CD8 ratios following treatment with ultrasonic hyperthermia. Biotechnol Ther. 1994;5(1–2):59–68. [PubMed] [Google Scholar]
- 105.Wu F, Wang ZB, Lu P, et al. Activated anti-tumor immunity in cancer patients after high intensity focused ultrasound ablation. Ultrasound Med Biol. 2004;30(9):1217–1222. doi: 10.1016/j.ultrasmedbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 106.Zhou Q, Zhu XQ, Zhang J, Xu ZL, Lu P, Wu F. Changes in circulating immunosuppressive cytokine levels of cancer patients after high intensity focused ultrasound treatment. Ultrasound Med Biol. 2008;34(1):81–87. doi: 10.1016/j.ultrasmedbio.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 107.Lewis GK, Jr, Guarino S, Gandhi G, et al. Time-reversal techniques in ultrasound-assisted convection-enhanced drug delivery to the brain: technology development and in vivo evaluation. Proc Meet Acoust. 2011;11:20005–20031. doi: 10.1121/1.3616358. [DOI] [PMC free article] [PubMed] [Google Scholar]