Abstract
This case report illustrates the role of using neuromuscular ultrasound to diagnose inclusion body myositis (IBM) in a patient who was previously diagnosed with polymyositis. Emerging studies have demonstrated the accuracy of MRI in detecting the selective involvement of the flexor digitorum profundus muscle in those with IBM. However, there have been only few reports on the use of ultrasound in diagnosing this condition. Our case demonstrates the benefit of using this ultrasonographic approach, which is simple, clear, inexpensive, painless and radiation-free, and provides another modality to assist in the evaluation of this sometimes difficult to diagnose condition.
Background
Inclusion body myositis (IBM) is the most common inflammatory myopathy, but it can be challenging to diagnose as electrodiagnostic testing can show mixed myopathic and neuropathic findings and muscle biopsy may not show classic pathology.1 We present a case in which neuromuscular ultrasound of the forearm assisted in the diagnosis of IBM in a patient previously diagnosed with polymyositis.
Case presentation
A man aged 80 years reported slowly progressive weakness in his legs. He saw his local neurologist within 6 months of onset and had two inconclusive nerve conduction studies and EMGs over the next 5-year period. He was then seen at an academic medical centre for a second opinion by a neuromuscular disease specialist, during which he reported difficulty using a cane when walking, mild difficulty swallowing and paresthesias in his feet. On examination, he had 4/5 strength in his flexor digitorum profundus muscles bilaterally. In the lower extremities, he was asymmetrically weaker in his left quadriceps (4/5 on left and 5/5 on right) and weaker distally with dorsiflexion and plantar flexion of both feet (2/5). He had mildly decreased sensation to temperature and vibration from toes up to his knees, and reflexes were slightly reduced throughout.
Investigations
Nerve conduction studies showed absent sural response and borderline tibial and fibular motor amplitudes without slowing. EMG revealed scattered abnormal spontaneous activity (fibrillations and positive sharp waves) in the arms and legs, along with a mixture of myopathic (short amplitude and duration) and neuropathic (tall amplitude and long duration) voluntary motor unit potentials. Recruitment was normal. A right deltoid biopsy demonstrated non-specific inflammatory cells invading non-necrotic muscle fibres. There were no rimmed vacuoles nor aggregates, and electron microscopy was not performed. He was diagnosed with ‘likely polymyositis’ by a neuromuscular disease specialist and referred to our institution for treatment (as he lived close to our medical centre).
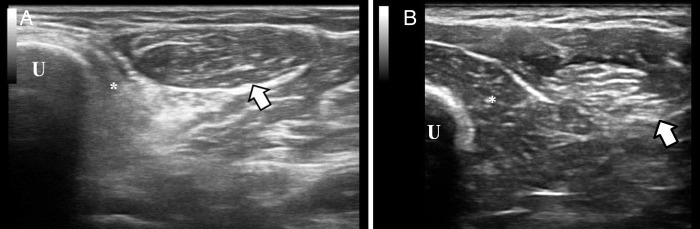
We conducted neuromuscular ultrasound of the left forearm, which showed significant atrophy of the flexor digitorum profundus muscles and normal size and echotexture of the flexor carpi ulnaris muscle (figure 1). This finding, in this clinical setting, made us highly suspicious of IBM. A cytosolic 5′-nucleotidase IA antibody was positive.
Figure 1.
(A) This ultrasonographic image is a cross-sectional view of the left forearm, obtained at approximately the upper third of the forearm. The ulna (U) is on the left side of the screen. The flexor digitorum profundus muscle (*) is directly adjacent to the ulna, and in this case, it is hyperechoic and atrophic. The flexor carpi ulnaris muscle (arrow) is adjacent to the flexor digitorum profundus muscle and has normal echotexture and size. Atrophic and hyperechoic flexor digitorum adjacent to normal flexor carpi ulnaris is the pattern consistent with IBM. (B) The same ultrasonographic view is presented in a healthy individual. No muscle atrophy is detected and the same structure as in image A are labelled.
Differential diagnosis
The patient's clinical presentation was consistent with a myopathic process. Initially, it was felt by a local neurologist that his presentation was consistent with polymyositis. However, on our evaluation, we felt that the distribution of his weakness (weakness in the flexor digitorum profundus and quadriceps) was more suggestive of IBM. Therefore, we did further testing as described above.
Outcome and follow-up
Despite the diagnosis of IBM, the patient requested a trial of steroids. After 4 months on 20 mg of prednisone daily, he noted no benefit and was tapered off, and no further immune modulation was pursued, given the diagnosis of IBM.
Discussion
Difficulty interpreting EMG and muscle biopsy in those with IBM can lead to misdiagnosis and inappropriate treatment, and therefore, new diagnostic modalities will assist in the evaluation of this condition. Over the past two decades, the role of imaging in the diagnosis of inflammatory muscle disease has been increasing.2 There have been advances in imaging across multiple modalities, including MRI, CT and ultrasound.3 MRI has been reported to detect selective involvement of the flexor digitorum profundus muscle in those with IBM with high accuracy.4 This finding has now been shown in ultrasound as well. Ultrasound is useful for imaging muscle abnormalities as it can be used to assess muscle and echogenicity.5 The low cost, ease of imaging at bedside and wide availability make ultrasound an appealing modality, particularly in cases of inflammatory and infectious muscle disease.5 6 Noto et al7 showed that those with IBM have atrophy and increased echogenicity in the flexor digitorum profundus muscles, which is in striking contrast to the normal appearance of the adjacent flexor carpi ulnaris muscle. When compared with other inflammatory myopathies and amyotrophic lateral sclerosis, they showed statistically significant (p<0.05) differences in flexor digitorum-to-flexor carpi ulnaris comparisons in regard to subjective assessments of muscle echogenicity, quantitative assessments of muscle echogenicity and quality measures of muscle thickness. Our case demonstrates the benefit of using this ultrasonographic approach, along with other diagnostic tests, in a patient with an unclear cause of weakness. This ultrasonographic technique is powerful because it is simple, clear, inexpensive, painless and radiation-free, and it provides another modality to assist in the evaluation of this sometimes difficult to diagnose condition.
Learning points.
Individuals over age 50 presenting with an inflammatory myopathy need to be assessed for inclusion body myositis (IBM), as identification of this condition is critical in guiding treatment decisions.
Ultrasound is painless, radiation-free and readily available, and it can be used effectively to diagnose IBM.
Individuals with IBM can be imaged with ultrasound in the proximal medial forearm, and pathognomonic findings will include atrophy of the flexor digitorum muscles with preservation of the flexor carpi ulnaris muscle.
Footnotes
Contributors: QV was the primary author of the manuscript and interpreted the results. MC involved in the revision process and gave the approval for submission.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Engel WK, Askanas V. Inclusion-body myositis: clinical, diagnostic, and pathologic aspects. Neurology 2006;66:S20–9. 10.1212/01.wnl.0000192260.33106.bb [DOI] [PubMed] [Google Scholar]
- 2.Machado PM, Dimachkie MM, Barohn RJ. Sporadic inclusion body myositis: new insights and potential therapy. Curr Opin Neurol 2014;27:591–8. 10.1097/WCO.0000000000000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo GP, Carrino JA. Skeletal muscle imaging and inflammatory mypathies. Curr Opin Rheumatol 2007;19:530–5. 10.1097/BOR.0b013e3282efdc66 [DOI] [PubMed] [Google Scholar]
- 4.Sekul EA, Chow C, Dalakas MC. Magnetic resonance imaging of the forearm as a diagnostic aid in patients with sporadic inclusion body myositis. Neurology 1997;48:863–6. 10.1212/WNL.48.4.863 [DOI] [PubMed] [Google Scholar]
- 5.Campbell SE, Adler R, Sofka CM. Ultrasound of muscle abnormalities. Ultrasound Q 2005;21:87–94. [PubMed] [Google Scholar]
- 6.Kumar MP, Seif D, Perera P et al. . Point-of-care ultrasound in diagnosing pyomyositis: a report of three cases. J Emerg Med 2014;47:420–6. 10.1016/j.jemermed.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 7.Noto Y, Shiga K, Tsuji Y et al. . Contrasting echogenicity in flexor digitorum profundus-flexor carpi ulnaris: a diagnostic ultrasound pattern in sporadic inclusion body myositis. Muscle Nerve 2014;49:745–8. 10.1002/mus.24056 [DOI] [PubMed] [Google Scholar]