Fig. 1.
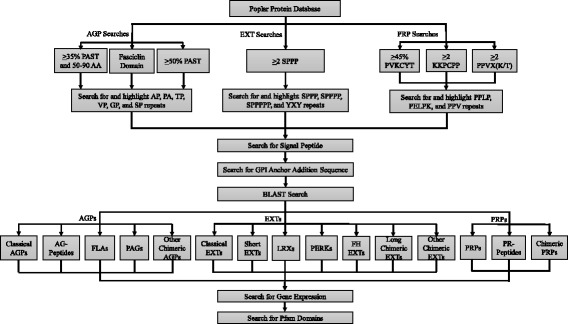
Workflow diagram for the identification, classification, and analysis of HRGPs (AGPs, EXTs, and PRPs) in poplar using a newly revised and improved BIO OHIO 2.0. Classical AGPs were characterized as containing greater than 50 % PAST. AG peptides were characterized to be 50 to 90 amino acids in length and containing greater than 35 % PAST. FLAs were characterized as having a fasciclin domain. Chimeric AGPs were characterized as containing greater than 50 % PAST coupled with one or more domain(s) not known in HRGPs. All AGPs feature the presence of AP, PA, TP, VP, GP, and SP repeats distributed throughout the protein. EXTs were defined as containing two or more SPPP repeats coupled with the distribution of such repeats throughout the protein; chimeric extensins, including LRXs, PERKs, FH EXTs, long chimeric EXTs (>2000 aa), and other chimeric EXTs, were similarly identified but were distinguished from the classical EXTs by the localized distribution of such repeats in the protein and the presence of non-HRGP sequences/domains, many of which were identified by the Pfam analysis; and short extensins were defined to be less than 200 amino acids in length coupled with the EXT definition. PRPs were identified to contain greater than 45 % PVKCYT or two or more KKPCPP or PVX(K/T) repeats coupled with the distribution of such repeats and/or PPV throughout the protein. Chimeric PRPs were similarly identified but were distinguished from PRPs by the localized distribution of such repeats in the protein. Other integrated functional modules include searching for the presence of a signal peptide to provide added support for the identification of an HRGP; the presence of a GPI anchor addition sequence for added support for the identification of AGPs, and BLAST searches to provide some support to the classification. Tissue/organ-specific expression data were also obtained for identified HRGPs to guide for future research
