Figure 3.
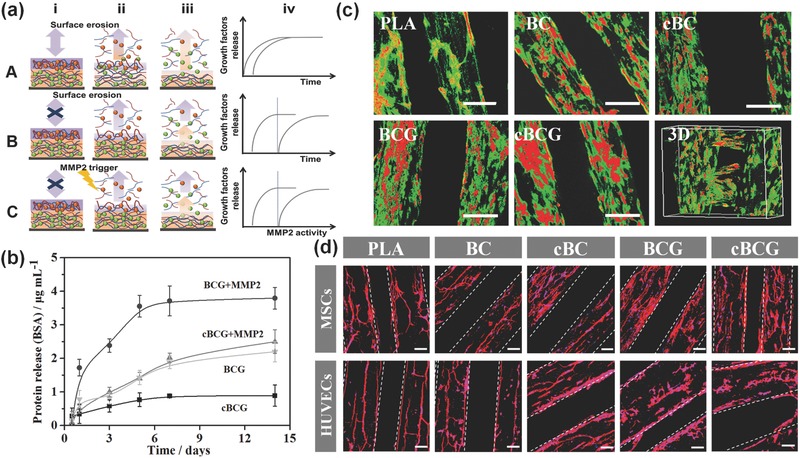
a) Illustration of the proposed assembly (i) and release (ii, iii) process of multilayer films without (A) and with (B) crosslinking, as well as our biologically inspired system (C), where the BMP2 (green spheres) and VEGF (red spheres) are loaded into films composed of PLL (blue) and MMP trigger‐cleavable Gel (red). Compared with traditional LbL film adsorption, crosslinking retain their stable immobilization and sequential release without highly inter‐diffusion. Moreover, surface erosion contributes film degradation where the therapeutic agent is released throughout the film, whereas biologically inspired system exhibits a controllable release behavior. The release profiles reflect the effect of crosslinking, and biologically inspired on kinetics of drug release (iv). b) Protein release profiles of nanocoating with BSA within 2 weeks. The cBCG could sustainably release up to 4 weeks (not shown in here). MMP2 was thought to trigger the cleavage of gelatin chain to controllably release growth factors. c) Confocal fluorescence images of hMSCs and HUVECs co‐culture on various scaffolds in a static culture condition for 5 days. hMSCs were labeled with cell tracker green, and HUVECs were stained with cell tracker red, respectively. The scale bars indicate 200 μm. The cBCG scaffold was also imaged as 3D scanning structure. d) Fluorescent images of hMSCs and HUVECs on the 3D bioprinted scaffolds with F‐actin (red) and nucleus (blue) staining in a static culture condition for 3 days. The hMSCs exhibited a well distributed spread on scaffold surface, while the HUVECs formed an aggregative microvascular networks. The scale bars indicate 100 μm.
