Figure 2.
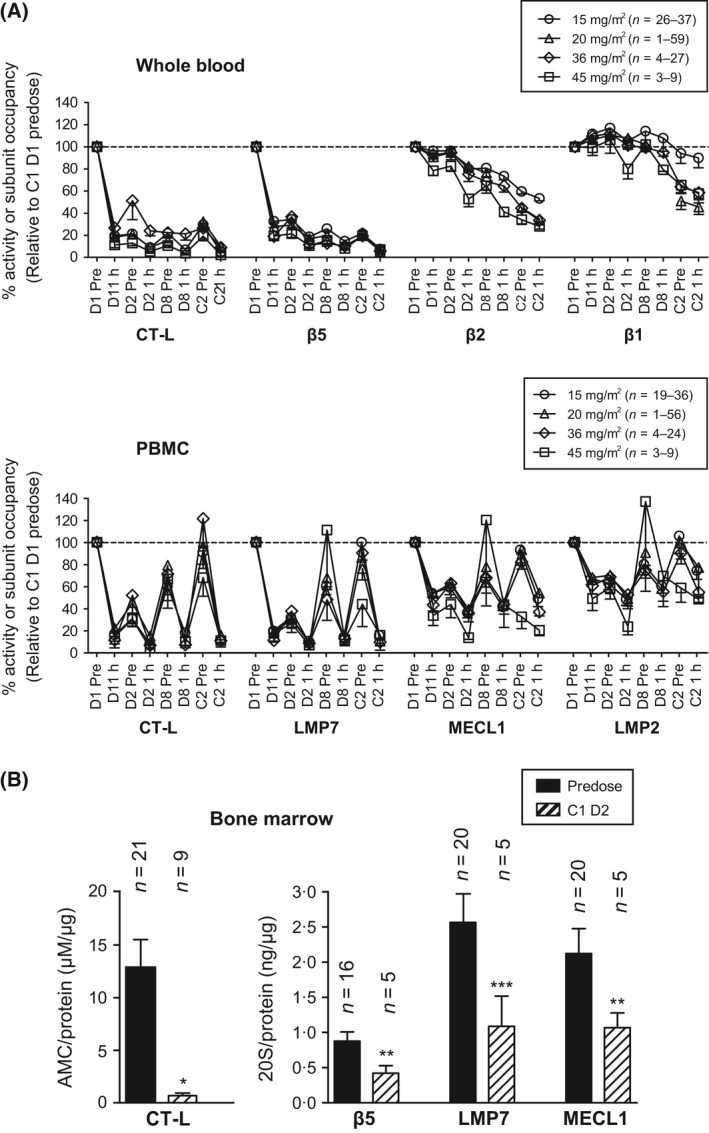
Repeat administration of carfilzomib results in sustained proteasome inhibition. (A) Patients received carfilzomib at 15, 20, 36 or 45 mg/m2 on days 1, 2, 8, D9, 15 and 16 of a 28‐day cycle. Whole blood and PBMC samples were taken prior to and 1 h after administration on days 1, 2 and 8 of cycle 1 and day 1 of cycle 2. Samples were analysed for proteasome activity and subunit occupancy as described in Fig 1 and were normalized to cycle 1, day 1 predose values. Data are presented as mean (±SEM) relative specific activity or occupancy. (B) Bone marrow samples from MM patients were taken during baseline screening and/or on cycle 1, day 2, 1 to 4 h after the second dose of carfilzomib. CD138+ cells and samples were analysed for proteasome activity and subunit occupancy. Data are presented as mean (±SEM) relative activity or subunit concentration. *P < 0∙001; **P < 0∙01; ***P < 0∙05 by a t‐test. C, cycle; CT‐L, chymotrypsin‐like; D, day; AMC, aminomethylcoumarin; LMP, low‐molecular mass polypeptide; MECL, multicatalytic endopeptidase complex‐like; MM, multiple myeloma; PBMC, peripheral blood mononuclear cell; SEM, standard error of the mean.
