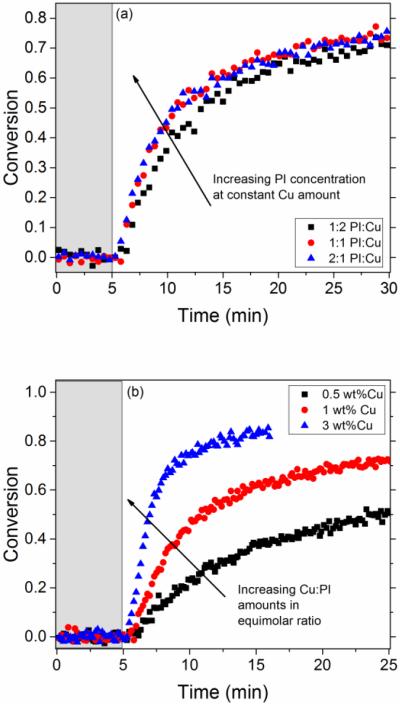
Figure 7.
Kinetic results in polymerizing systems using Irgacure 819 and CuCl2-PMDETA along with monomers 1 and 2 as the reactants and at a constant intensity of 10 mW/cm2 of 405 nm wavelength light and a sample thickness of 0.12 mm. The grey, shaded area represents the time (5 min) before the light was turned on. (a) Threshold value in photoinitiator applies in network forming systems. When the concentration of photoinitiator is increased beyond half that of copper(II), from a 1:2 mol ratio Irgacure 819:CuCl2-PMDETA (square) to an equimolar ratio (circle) and then a 2:1 mol ratio (triangle), there was little increase in the reaction rate. (b) When the concentration of photoinitiator and copper(II) where increased in equimolar concentrations from a 0.5 wt% of Copper(II) (square) to 1 wt% Copper(II) (circle) to 3 wt% ratio (triangle)., the rate of the reaction significantly increases.