Abstract
Caloric vestibular stimulation (CVS) to elicit the vestibulo-ocular reflex has long been used in clinical settings to aid in the diagnosis of balance disorders and to confirm the absence of brainstem function. While a number of studies have hinted at the potential therapeutic applications of CVS, the limitations of existing devices have frustrated that potential. Current CVS irrigators use water or air during short-duration applications; however, this approach is not tenable for longer duration therapeutic protocols or home use. Here, we describe a solid-state CVS device we developed in order to address these limitations. This device delivers tightly controlled time-varying thermal waveforms, which can be programmed through an external control unit. It contains several safety features, which limit patients to the prescribed waveform and prevent the potential for temperature extremes. In this paper, we provide evidence that CVS treatment with time-varying, but not constant temperature waveforms, elicits changes in cerebral blood flow physiology consistent with the neuromodulation of brainstem centers, and we present results from a small pilot study, which demonstrate that the CVS can safely and feasibly be used longitudinally in the home setting to treat episodic migraine. Together, these results indicate that this solid-state CVS device may be a viable tool for non-invasive neuromodulation.
Keywords: Caloric vestibular stimulation, neuromodulation, migraine, cerebral blood flow, brainstem
This paper describes a non-invasive neuromodulation device based on caloric vestibular stimulation (CVS). The device architecture is described and the results of two pilot studies are discussed. The first study suggests that the CVS device can be used successfully in the home by patients with episodic migraine headaches. The second study shows evidence that the CVS device may entrain a brainstem pacing center, which is a part of the autoregulatory system, and which has been studied in the context of migraine pathology.

I. Introduction
Caloric vestibular stimulation (CVS) is a widely used technique developed by Robert Barany more than a century ago [1], and is commonly used to diagnose balance disorders or confirm absence of brainstem function. Historically, water or air irrigators have been used to warm or cool the external auditory canal of patients. Both warming and cooling temperature changes lead to density changes in the endolymphatic fluid in the semicircular canals and create convection currents which result in cupular deflection, change in the tonic firing rate of the vestibular nerves and elicit the vestibulo-ocular reflex or horizontal nystagmus. Though there is still debate about the totality of effects that caloric stimulation has on the vestibular organs, the thermoconvection model originally described by Barany and Wittmaack [2] seems to account for the majority of the induced changes (at least within earth’s gravity [3]).
CVS induction has been associated with release of a number of neurotransmitters including serotonin [4], histamine [5], acetylcholine [6] and GABA [7]. Additionally, the modulation of various networks and nuclei in the brain by CVS including the basal ganglia [8] cerebellum, brainstem, insula [9], hypothalamus [10], thalamus [11], hippocampus [9] and prefrontal cortex [12] suggests significant potential for CVS to modulate both motor and non-motor functions.
Despite the evidence for widespread activation of neural circuits [13] and a long history to support the clinical safety of CVS, investigation into its potential therapeutic efficacy has been limited. Several groups have demonstrated acute benefits of CVS with hemispatial neglect patients [14]–[20], and a number of studies have explored the therapeutic potential of CVS for pain mitigation [21]–[23]. However, all of these studies have suffered from low patient numbers, minimal details about the CVS protocol, no longitudinal treatment, and cursory follow-up, if any. The main impediment to progress for CVS as a mechanism for neuromodulation and/or therapeutic approach has been the lack of a device design suited for prolonged treatments and home use [24]. To address this deficit, we developed a novel, solid-state device for delivering CVS. We describe the results herein and provide evidence that time-varying thermal CVS waveforms elicit physiological changes in cerebral blood flow velocity, which are consistent with neurostimulation of brainstem centers. Furthermore, we demonstrate the feasibility and safety of longitudinal use of the device for the treatment of episodic migraine.
II. Materials and Methods
A. Device
The CVS device (Fig. 1) is easily operated and consists of a headset, fashioned like music headphones, with aluminum earpieces to conduct heat and a control unit that powers the device and allows for patients to start treatments. The temperature in each ear insert is separately controlled allowing for a wide variety of time-varying waveforms. However, the device has fail-safe and patient-lockout protections, so the patient can only activate the neuromodulation protocols and number of daily treatments prescribed on the SD cards inserted into the control unit.
FIGURE 1.
CVS device headset and control unit.
The CVS device is based on a Peltier unit, an array of diodes comprised of n-type/p-type semiconductor junctions comprised of bismuth telluride (see Fig. 2). The Peltier module used in the CVS device is composed of an array of diodes in a cast epoxy cube to reduce susceptibility to shear stress (TE Technology, Traverse City, MI). When direct current is passed in one direction through the array, one side heats up with respect to the other. Reversing the direction of current flow also reverses the temperature gradient across the array. A pulse-width-modulation algorithm is used to power the Peltier devices so that the actual temperature, as measured by a thermistor in the earpiece, matches the waveform target temperature. The anodized, aluminum earpiece is attached to one side of the Peltier array and an aluminum heat sink is attached to the other. The Peltier device mates to the heat sink via a solid aluminum standoff. Fig. 2b shows an enlarged view of the connections to the Peltier device as well as the location of a thermistor temperature sensor, which is used in the control circuit that sets the temperature of the earpiece. The groove in the ear insert is designed to allow for pressure equalization within the ear canal. The earpiece is covered on its lower base by an ethylene-vinyl copolymer skirt that works to thermally insulate the earpiece from the outer portions of the ear canal and concha. Fig. 3 shows a thermograph of the components in Fig. 2b.
FIGURE 2.
(a) Exploded view of CVS headset. (b) Schematic showing the principal elements of the earpiece.
FIGURE 3.
Infrared thermographs show the temperatures of the earpiece and heat sink during a square wave thermal waveform with a minimum temperature of 15 °C and a maximum temperature of 42 °C. Black is cold, white is hot.
The thermistor in each earpiece samples the temperature at the tip at a rate of 4 Hz. The thermistor provides feedback to the control circuit that sets the temperature of the earpiece. The heating and cooling systems are deactivated if either thermistor detects temperatures 1 °C below or above the minimum and maximum temperatures defined by the thermal waveforms, respectively, or if a thermistor fails. The temperature control circuit accesses a temperature calibration file matched to a specific thermistor whenever the system is powered on. The device will display an error and will not run a treatment if no calibration file is found. Additionally, because the warming and cooling functions are driven by the system software, any potential failure of the system software would result in inactivation of the device.
The thermistor-recorded temperature is logged with each treatment thus providing a mechanism to confirm treatment compliance. Because the thermal load of a subject can lead to a small drop in thermal efficiency later in the run (thus creating a small divergence between the measured and targeted temperature, see Fig. 4), the temperature log can also be assessed to determine whether the subject actually wore the headset during any particular treatment.
FIGURE 4.
Example of target and actual thermal profiles of the sawtooth time-varying thermal waveform used in this study.
The device was manufactured by Anuva Innovations (Morrisville, NC) under ISO 13485 compliant protocols. All hardware, software and safety features of the device were tested according to ISO 60601 standards by an independent testing laboratory, Intertek (Atlanta, GA), and the biocompatibility of device materials was independently confirmed under ISO 10993 by Toxikon (Bedford, MA).
B. Mode of Action
Proper CVS induction relies on good thermal transfer between the earpieces and the temporal bone. Heat flows around the middle ear space to the inner ear and the vestibular labyrinth. The horizontal semicircular canal (hSCC) actually protrudes slightly into the middle ear space and is the structure most immediately and strongly affected during CVS [25]. The base of the earpiece is insulated with rubber to prevent unwanted heat transfer from other than the shaft and tip. There is a wide variation in tortuosity of the fleshy part of the ear canal in adults, but the bony canal, which terminates at the tympanic membrane, is similar in all adults [26]. The earpiece tip is sized so that it cannot enter the bony canal, but a good fit will ensure that the tip is abutted to the bony canal entrance. Though small variations in the fitting of the headset may exist across patients or day-to-day within a given patient, CVS is intrinsically well targeted since the thermal stimulus only stimulates the vestibular organs.
With conventional irrigation-based CVS, there is a period of rapid temperature change followed by a constant temperature epoch and then a slower recovery to body temperature as the irrigation stops. Plotted as a time-varying waveform, this would be a very high slew rate change to a plateau followed by a nonlinear ramp back to 37 °C. By contrast, the CVS device allows for defined, time-varying waveform shapes (for an example, see Fig. 4) and has a slew rate limited by the thermal capacity of the heat sink, but it can achieve roughly 40 °C/minute for heating and 20 °C/minute for cooling. Notably, the CVS device can repeat a waveform pattern to create cyclic CVS. Though the amplitude of the thermal waveform is reduced and the sharp vertices of a sawtooth waveform pattern are smoothed to a shape resembling a sine wave (due to the thermal conduction properties of the temporal bone) the frequency is preserved at the inner ear target. Making exact predictions about temperature profiles or tonic firing rates is not possible due to the inter-individual variations in pneumatization of the bone [27], blood flow, the quality of the fit of the earpiece, etc.
While dizziness and nausea have been associated with conventional irrigation-based CVS, tolerability can be improved and unwelcome side effects can be mitigated by avoiding temperature extremes [1] and by slowing the slew rate. Notably, although primary endpoints will not be discussed in this paper, the waveform shown in Fig. 4 has been used in a number of studies to investigate the therapeutic efficacy of the CVS device for a number of neurological and metabolic conditions. There were only minimal adverse effects in these studies (and no serious or unexpected adverse effects). Additionally, in over 100 patients who have participated in other studies using the time-varying CVS waveform shown in Fig. 4, only 4 patients have reported experiencing dizziness and/or nausea (transient in all cases) that could have potentially been related to treatment, and no serious or unexpected adverse events were reported. Of the 4 patients who experienced dizziness/nausea during the study, no patient’s symptoms were conclusively linked to use of the device (Black and Rogers, unpublished observations).
In addition to minimizing risk of dizziness and nausea, the precise temporal control of the thermal waveforms allows the device to overcome the therapeutic limitation of SCC adaptation which is observed during constant temperature CVS [28], [29]. It is important to note that the diminution of nystagmus to constant temperature CVS is due to a physical adaptation of ciliary structures, which respond to the motion of the cupula, rather than the fatigue of afferent nerves. In fact, Bagnall et al. [30] found that the vesicle structure in the hair cell synapses has evolved to reduce fatigue. By varying the temperature applied to the temporal bone during CVS, adaptation can effectively be avoided, thus allowing for a therapeutic session limited only by the endurance of the patient. Finally, having independently controlled caloric earpieces allows for “unyoking” the left and right labyrinths, enabling non-physiological stimulation to be delivered.
C. Measuring CVS-Induced Changes in Cerebrovascular Dynamics
The effects of CVS on cerebrovascular dynamics were measured using transcranial Doppler sonography (TCD) of intracranial blood vessels using a Sonoara TCD system (Natus Medical, Pleasanton, CA). Following Duke University IRB approval, the basilar artery (BA) of a single subject was insonated through the suboccipital acoustic window over the course of three CVS treatments. The Gosling Pulsatility Index (PI), a measure of cerebrovascular resistance defined as [(peak systolic velocity - end diastolic velocity)/ mean cerebral blood flow velocity] constituted the primary measure of cerebrovascular dynamics. Eight nearest-neighbor averaging was performed to smooth the curves. Heart rate (HR) from the TCD signal was also assessed.
Successful insonation of the BA was judged by real-time monitoring of the return signal on the TCD unit. A 2 MHz probe was used and the sampling rate was 0.71 seconds. The ultrasound probe was coated with a viscous acoustical coupling gel and held, by hand, at the base of the skull. The PI time series data was transformed, using a Fast Fourier Transform (FFT) algorithm (StatPlus, AnalystSoft, Walnut, CA) into a frequency space representation to better highlight the spectral power distribution of the observed oscillations. Three minute long segments of the time series were transformed with no additional digital filtering. Data is presented on a scale from 0.3 to 9.0 cycles per minute (cpm) to provide better resolution of the primary observed spectral components.
D. Pilot Study to Investigate Feasibility of Home-Use CVS for the Prevention of Episodic Migraine Headache
Following Duke University IRB approval, a small pilot clinical study with episodic migraine patients was conducted primarily to establish safety and feasibility for use of this device in the home environment. However, the pilot study was also designed to evaluate different CVS thermal waveforms and gather preliminary evidence of efficacy. Three patients met the following inclusion criteria and were thus enrolled in the study:
-
•
A history of at least four, and not more than fourteen total monthly headache days of which between four and nine were migraine headache days. Patients were permitted continued access to migraine abortive medications. Patients were being treated at the Duke University headache clinic.
-
•
A history of some responsiveness (incomplete) to at least one and a maximum of two prophylactic pharmaceutical therapies (utilized concurrently).
-
•
Subject were at least 18 years of age.
Exclusion criteria:
-
•
Individuals who were pregnant, who had a history of cardiovascular disease, who worked night shifts or who had vestibular migraine, menstrual migraine, post-traumatic migraine, a history of unstable mood disorder or unstable anxiety, moderate or greater hearing loss or a history of traumatic brain injury.
-
•
A history within the last six months of narcotic or barbiturate use or experience of one or more analgesic rebound headaches.
Study parameters:
-
•
Patients self-reported baseline migraine headache burden.
-
•
Patients used the CVS device for a period of 6 weeks, and treatment with the CVS device consisted of twice daily, roughly 18 minute long sessions (active only, no placebo).
-
•
Patients maintained a pain diary during the treatment period as well as a 4 week post-treatment observation period where they self-reported headache pain burden using visual analogue scale for pain (0-10 point subjective pain reporting). The number of headache days was binned over 2-week intervals and was used as a preliminary measure of efficacy.
-
•
Balance was tested using the Berg scale [31] prior to treatment and at the end of the 6-week treatment period.
-
•
Patients were required to report any adverse events.
One male and two female patients were recruited into the study. Patient #1 had a device that delivered an in-phase square wave pattern to both ears with a 2-minute period, transitioning from 17 to 42 °C. The other two patients had devices that delivered the sawtooth pattern shown in Fig. 4. For these two patients, the ears receiving the warm and cold waveforms were switched on a daily basis to mitigate the hypothetical risk of unintentionally creating a baseline vestibular asymmetry after the treatment period. Both the square and the sawtooth thermal waveforms were designed to avoid adaptation of the vestibular response. The square wave program delivered in-phase thermal stimuli to both ears. This waveform choice was designed to mitigate side effects by negating the asymmetric effect concomitant with unilateral CVS administration.
III. Results
A. CVS Treatment Modulates Cerebrovascular Dynamics
Vestibular stimulation, including CVS, has previously been shown to affect cerebrovascular dynamics [32]–[34]. To demonstrate that our device provides substantive stimulation of the vestibular system, we measured the effects of treatment on PI using TCD sonography over the course of a CVS treatment. Because the thermoconvective effect of CVS is maximized when the horizontal semicircular canal (hSCC) is in the vertical orientation, we first evaluated the effects of CVS on cerebrovascular dynamics when the subject was supine on a ~22° wedge pillow, a position which puts hSCC in the optimal orientation for CVS [35].
In this position, the time-varying sawtooth thermal waveform (shown in Fig. 4) induced strong oscillations in PI after ~4-5 minutes of CVS treatment (Fig. 5a). These oscillations in PI continued throughout the remainder of the CVS treatment and for approximately 7 additional minutes during the post treatment period. Power spectra analysis of the PI time course show prominent peaks clustered around 1.5 cpm for the mid to late-phase CVS treatment as well as the early post-treatment period as compared to baseline (Fig. 5b). Also, peaks ~3.0-3.3 cpm (possible harmonic) were observed during these periods. In the 21-24 minute segment (early post-CVS), the peak power increased, suggesting a possible entrained resonance. Notably, the power spectra for the last 3 minutes of the time series resembles that of the baseline suggesting that PI had returned to pre-CVS values.
FIGURE 5.
(a) PI time course, (b) PI power spectrum, (c) HR time course and (d) HR power spectrum show physiological effects of time-varying CVS treatment when the subject is supine on a 22° wedge pillow. (e) Time course data for time-varying CVS in the supine position shows an anti-phase relationship between PI (purple) and HR (green), with oscillations in PI occurring first. (f) PI time course and (g) PI power spectrum when the subject is bent forward in a null position. The subject did not complete the entire post CVS time course during this recording. (h) PI time course and (i) PI power spectrum for constant temperature CVS (17 °C) when the subject is supine on a 22° wedge pillow. For panels a-d and f-i, baseline (black), CVS treatment (red), post-CVS treatment (blue).
Evaluation of heart rate variability also showed that CVS treatment induces significant oscillations in heartbeats per minute (Fig. 5c). Similar to the case for PI, power spectra analysis for heart rate also revealed significant peaks around 1.5 cpm and ~3.0 cpm, (Fig. 5d), although peaks at other frequencies were also present. Notably, the HR oscillations demonstrated an anti-phase relationship to the PI oscillations (Fig. 5e). The anti-phase relationship was particularly prominent during late-phase CVS. Additionally, the onset of the HR oscillations occurred after the first peak in PI and indicating that this entrainment of HR could have been a secondary compensatory response to the fluctuations in PI.
To rule out the possibility that the observed CVS-induced changes in cerebrovascular dynamics resulted from a direct thermal effect on vestibular afferent firing (i.e. that warming or cooling temperatures reaching the nerve itself, affect the tonic firing rate), we repeated this experiment (in the same subject on a separate day) with the subject sitting up in a slightly forward bent position which leaves the hSCC in the horizontal orientation. We termed this the null position because the horizontal orientation of the hSCC should abolish the potential for changes in temperature to generate thermoconvective currents. CVS treatment in the null position did not induce oscillations in PI (Fig. 5e) or HR (data not shown) and there were no observed peaks in the power spectra that were significantly different from baseline (Fig. 5g). The lack of substantive PI or HR oscillations while the subject was in the null position also rules out the possibility, however unlikely, that the CVS-induced changes in cerebrovascular dynamics observed when the subject was in the supine position were somehow mediated by a placebo-effect due to subjective sensations of warming and cooling temperatures in the ears as this sensory experience remained constant throughout.
Finally, to examine the significance of our time-varying thermal waveform, we next evaluated whether PI oscillations could be induced by a constant temperature CVS waveform. For this, the subject was supine on the ~22° wedge pillow and a constant temperature (17 °C) waveform was applied to both ears for 21 minutes. Similar to the time-varying waveform in the null position, constant temperature CVS did not result in oscillations in PI (Fig. 5f) or HR (data not shown). Although further studies will be required to validate these findings, together, these results provide physiological evidence for substantive and prolonged stimulation of the vestibular system by time-varying CVS device treatment.
B. Feasibility of Home-Use CVS for the Prevention of Episodic Migraine Headache
All 3 patients completed the 6-week treatment period and 4-week follow up. Treatment compliance was above 80% in all cases (i.e. patients completed at least 67 of the 84 total treatments over the 6 weeks). Significantly, no patient reported adverse events that could be attributed to use of the CVS device either during or after the treatment period. There were no observable changes in balance (as measured using the Berg balance test) between the baseline and the 6-week post treatment evaluation. Patient evaluations revealed that twice-daily treatments at home were quite manageable. Specific comments on the device design generally related to inconvenience of the cabling leading from the headset to the control unit. However, no patient reported significant procedural inconvenience during treatments.
Although the primary intent of this pilot study was to gather data on the feasibility and safety of using this portable CVS system in the home setting, evaluation of the of the daily diaries revealed that the number of headaches after 6 weeks of CVS therapy was lower than the self-reported headache frequency prior to entering the study for all 3 patients (Fig. 6). These results suggest that CVS treatment may provide therapeutic relief for those suffering from episodic migraine headaches and will be validated in a larger scale research clinical trial.
FIGURE 6.
The number of headaches reported over the course of the treatment and post-treatment observation periods binned into 2 week intervals.
IV. Discussion and Conclusions
Irrigation-based CVS has been used for over a century both to diagnose balance disorders and confirm loss of brainstem function. Despite a long-standing history of clinically efficacy, the therapeutic potential for caloric-based vestibular stimulation has remained largely unexplored. The primary impediments for evaluating the therapeutic potential of CVS stem from the lack of compatibility of irrigation-based calorics for self-administration in the home and the short duration of vestibular stimulation which can be achieved using constant-temperature CVS. The solid-state CVS device, described herein, overcomes these limitations of traditional irrigation-based calorics and provides an unprecedented opportunity to evaluate the therapeutic potential of CVS treatment for a number of neurological and metabolic disorders.
One of the most significant advances the device affords over traditional irrigation-based CVS is the ability to provide time-varying thermal waveforms. In this study, we show that time-varying CVS has a qualitatively different effect on cerebrovascular physiology than does constant temperature CVS. More specifically, time-varying, but not constant-temperature CVS induces significant oscillations in PI (as well as HR) after 4-5 minutes of treatment (Fig. 5). Analysis of the PI spectral peaks during time-varying CVS indicates that the oscillations occur at 40 seconds (and 20 second harmonic) intervals. While these intervals do not match the periods of the warm (1.3 minutes) and cold (2.3 minutes) waveforms, they do fall within the periodicity range of B waves (sharp rhythmic sawtooth-like oscillations in intracranial pressure) [36] which are thought to be autoregulatory reactions to changes in arterial blood pressure. B waves occur at frequencies of 0.5 to 3 oscillations per minute) [36], [37], and due to their slow time constant, are thought to provide a complimentary mode of autoregulation to the rapid autoregulatory responses that are required when going from seated to standing, for example. This slower mode of autoregulation likely allows for compensation against baseline shifts that could increase intracranial pressure over time.
Although much remains unknown about the precise circuit mechanisms that allow for B wave generation, previous studies have provided evidence for a monoaminergic B-wave pacing center in the pons [37], an area that receives direct innervation from the vestibular nuclei in the brainstem [38]. What seems clear from the PI data is that the observed oscillations resulted from vestibular stimulation (since there was an orientation dependence) and that time variation of the thermal waveforms was necessary (since the constant temperature CVS run did not generate oscillations). The periods of the applied thermal waveforms did not exactly match the observed oscillation periods, but it is well known that an oscillator can be driven at a non-resonant frequency [39]. When CVS stimulation stopped, the immediate post-CVS spectra sharpened to a single primary peak at ~1.5 cpm (plus a harmonic). Because the PI oscillations fell within the periodicity for B waves (0.5 – 3.0 cpm [36], [40]), it is possible that the PI oscillations resulted from B wave activity. That is, the time-varying CVS may have entrained the pontine structures responsible for B-wave pacing. Indeed, the finding that there was a significant increase of spectral power at 1.5 cpm when the driving force of CVS stopped (post-CVS period) supports the hypothesis that entrainment occurred.
Although speculative, the evidence that time-varying CVS seemed to entrain B wave pacing, could provide evidence to suggest a potential mechanism of action for CVS treatment of migraine headaches. More specifically, Sliwka et al. [40] found that migraineurs exhibit a greater coefficient of variation in B wave amplitude compared to healthy controls and patients with tension headaches. The authors concluded that this was evidence of dysfunction of a brain stem monoaminergic system in migraine. Further investigation into the modulation or entrainment of B wave pacing by time-varying CVS and its potential role in the therapeutic effects for migraine prevention will be an exciting area of future research.
B wave flow abnormalities, if they are truly associated with migraine, are not in and of themselves thought to be causal in the disease. While migraine is associated with vascular flow abnormalities, neuroimaging findings support the hypothesis that migraine is a neurological disorder involving brainstem dysfunction. During acute attack, brain activation is observed in the dorsal pons and also the dorsal midbrain [41]. This activation persists after pain is controlled through abortive medication; however, no differences in activation of these regions are observed interictally [42].
How might CVS-driven neuromodulation affect the brainstem origins of migraine? Functional imaging studies of irrigation CVS have demonstrated widespread activation patterns in the brain, starting in the vestibular nuclei in the dorsal pons and medulla. For a review, see Dietrich and Brandt [13]. Although neuroimaging data evaluating patterns of brainstem activation with CVS have been limited due to the difficulty of achieving anatomic resolution with these techniques, a number of tracing studies in animal models have demonstrated that the vestibular nuclei are extensively connected to numerous brainstem regions implicated in migraine headache including the periaqueductal gray, the parabrachial nucleus, the locus coeruleus, the reticular formation, the dorsal spinal and mesencephalic trigeminal nuclei, and the dorsal raphe nuclei [43], [44]. This extensive anatomic connectivity of the vestibular nuclei with key brainstem regions implicated in migraine headache, together with our evidence for substantive and prolonged stimulation using time-varying thermal CVS waveforms and our preliminary evidence from the episodic migraine pilot (showing the safety, feasibility and potential efficacy of longitudinal use of the device) has given us incentive to follow up this work with prospectively powered, randomized, placebo-controlled clinical trial (NCT01899040; http://www.clinicaltrials.gov). That trial seeks to gather data in support of an application for regulatory clearance by the U.S. Food and Drug Administration.
Titration of CVS for therapy is still phenomenological. The temperature ranges and slew rates of the two different thermal waveforms used in the migraine pilot evaluation were selected to minimize adverse events. Importantly, these two waveforms were well tolerated in the migraine pilot study and in a larger scale study. Additionally, in our waveform selection, we sought to “balance” CVS delivery so that unintentional (hypothetical) biasing of baseline vestibular function did not occur. To balance CVS, either the waveforms delivered to the two sides were matched or asymmetric waveforms were alternated over time to achieve parity. In future studies, we will seek to establish the physiological sequelae, safety and tolerability of other thermal waveforms.
We expect that individualized titration for patients using CVS therapy is likely to be necessary. Whether evaluation of cerebrovascular dynamics in response to CVS waveforms may be beneficial in gauging the potential for therapeutic efficacy will be an interesting area for future studies. However, it is important to note that given the inherent selectivity of CVS induction (that is, only the vestibular organs respond to caloric stimulation), treatments should be reproducible. By contrast, two other neuromodulation devices under development for migraine treatment, transcranial magnetic stimulation [45] and cranial electrotherapy stimulation or transcranial alternating current stimulation [46] (which was recently found to have significantly weaker stimulation that the original models predicted [47]), are inherently dependent on patients placing the devices consistently for each treatment session. Inconsistencies with device placement may result in variability in either stimulation intensity or brain regions activated by treatment, and thus are significant limitations of these neuromodulation approaches [48].
In addition to having potential therapeutic efficacy for migraine headaches, we hypothesize that CVS treatment with our device may be beneficial for a number of clinical disorders. Indeed, a pilot study (using our device) demonstrated that with time-varying CVS treatment, two minimally conscious patients, transitioned from involuntary to voluntary behavior and that this effect was time-locked to active treatment [49]. Additionally, a case study in a Parkinson’s disease patient demonstrated time-locked and persistent improvements in both motor and non-motor symptoms with time-varying CVS treatment [50]. The findings for durable gains in these two studies, even after cessation of CVS treatment, suggest that CVS therapy may induce mechanisms of neuroplasticity. Additionally, these two studies highlight a range of therapeutic potential for CVS. Furthermore, the comorbidity among balance disorders and anxiety disorders (as well as migraine), reviewed by Balaban et al. [38], suggest the vestibular system may play a significant role in regulating psychiatric function and mood. Thus, vestibular neuromodulation may provide a novel therapeutic approach for the treatment of anxiety and mood disorders.
Finally, evidence exists which suggest that another form of vestibular stimulation, galvanic vestibular stimulation (GVS), may improve postural stability and gait in Parkinson’s disease and balance disorders [51], [52] as well as sensory perception after stroke [53]–[56]. Thus, vestibular stimulation through our CVS device may also provide therapeutic efficacy in these realms. However, there are a number of qualitative differences between CVS and GVS. While GVS is believed to primarily affect irregular afferent neurons of the 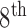 cranial nerve, CVS affects both regular and irregular neurons. Regular neurons, which outnumber irregular neurons by about 3:1, seem to play a more prominent role in fine/learned motor skills [57] whereas irregular neurons are thought to be involved in detecting changes in acceleration [58], [59]. In light of these differences, it is unsurprising that neuroimaging studies have found different patterns of cortical activation in response to CVS and GVS [60]. Finally the kinetics of activation significantly differ between these two modes of vestibular stimulation. In CVS, the period of a waveform is limited by the thermal conduction time of the temporal bone, whereas GVS can be implemented up to many 10’s of Hz. However, it is important to note that the actual firing pattern created by time-varying CVS in the vestibular nuclei is complex. Regular neurons have an equilibrium firing rate of ~100 Hz and cold CVS lowers the firing rate whereas warm CVS raises it. A temperature ramp, therefore, creates an increasing or decreasing frequency signal, often termed a “chirp.” Thus, even though the frequency of a CVS thermal waveform is significantly less than 1 Hz, the induced firing rate extends for many 10’s of Hz above and below the equilibrium firing rate. Furthermore, each ear can be stimulated independently, leading to a highly complex frequency modulation space in the brainstem.
cranial nerve, CVS affects both regular and irregular neurons. Regular neurons, which outnumber irregular neurons by about 3:1, seem to play a more prominent role in fine/learned motor skills [57] whereas irregular neurons are thought to be involved in detecting changes in acceleration [58], [59]. In light of these differences, it is unsurprising that neuroimaging studies have found different patterns of cortical activation in response to CVS and GVS [60]. Finally the kinetics of activation significantly differ between these two modes of vestibular stimulation. In CVS, the period of a waveform is limited by the thermal conduction time of the temporal bone, whereas GVS can be implemented up to many 10’s of Hz. However, it is important to note that the actual firing pattern created by time-varying CVS in the vestibular nuclei is complex. Regular neurons have an equilibrium firing rate of ~100 Hz and cold CVS lowers the firing rate whereas warm CVS raises it. A temperature ramp, therefore, creates an increasing or decreasing frequency signal, often termed a “chirp.” Thus, even though the frequency of a CVS thermal waveform is significantly less than 1 Hz, the induced firing rate extends for many 10’s of Hz above and below the equilibrium firing rate. Furthermore, each ear can be stimulated independently, leading to a highly complex frequency modulation space in the brainstem.
From a device usage perspective, the CVS device described herein is a particularly innovative method for vestibular stimulation because it can be administered with little or no technical expertise. It simply requires an earpiece to be fitted within the external ear canal (like a headphone or ear plug) and a pre-set thermal stimulus is then generated from a small stimulation unit. Unlike other rehabilitation techniques, no dedicated space, technical staff or lengthy setup procedures are required. A primary challenge for successful migraine prophylaxis (or any preventative treatment) is compliance with therapy over time, and thus, ease-of-use is a crucial element of design.
In our pilot study with migraineurs, compliance was excellent, and no adverse effects were reported. Although anecdotal, measures of headache reduction after treatment with the CVS device suggest the importance of performing an adequately powered, randomized controlled trial. Such a trial will be the starting point for actual translational work addressing the needs of migraineurs, and will also lay groundwork for evaluating other therapeutic applications of the CVS device.
Acknowledgment
The authors are grateful to Lanty L. Smith (Scion NeuroStim, LLC) for intellectual and funding support for this study.
 Note: the CVS device described in this paper is for investigational use only.
Note: the CVS device described in this paper is for investigational use only.
Biographies

Robert D. Black received the Ph.D. degree in electrical engineering from the University of Illinois, Urbana, in 1984. He was a Researcher with the General Electric Research and Development Center and an Assistant Professor of Radiology with Duke University, and an Executive/Co-Founder of several medical device companies. He has been an Adjunct Professor with the Joint Department of Biomedical Engineering, The University of North Carolina, Chapel Hill, and North Carolina State University, Raleigh. He is currently a Principal with Scion NeuroStim, a development stage neuromodulation company. Dr. Black is a fellow of the American Institute for Medical and Biological Engineering.

Lesco L. Rogers received the M.D. degree from the Geisel School of Medicine, Dartmouth College, New Hampshire, in 1990. He completed a residency in anesthesiology at Robert Wood Johnson University Hospital and an interventional pain management fellowship at Georgetown University. He is currently an Adjunct Clinical Assistant Professor in the Department of psychiatry with Duke University and Wake Forest University. He is currently a Chief Scientific Officer of Scion NeuroStim. His research interests include migraine headaches, Parkinson’s disease, and mood disorders.

Kristen K. Ade received the Ph.D. degree in neuroscience from Georgetown University in 2008. She completed a post-baccalaureate fellowship with the Laboratory for Integrative Neuroscience, National Institute on Alcohol Abuse and Alcoholism in 2002. She completed a post-doctoral fellowship with the Departments of Neurology and Neurobiology, Duke University in 2016. She is currently the Chief Executive Officer of NeuroVision Consulting, LLC and serves as a Scientific Consultant for Scion NeuroStim.

Heather A. Nicoletto received the B.S. degree in ultrasound and vascular option from the Oregon Institute of Technology in 2000. She was a Technologist with the Cerebrovascular Laboratory, Harborview Medical Center in 2005 when she moved to North Carolina. She is currently the Manager of the Neurovascular and Neuromuscular Laboratories under Neurodiagnostic Services with Duke University Hospital.

Heather D. Adkins received the M.D. degree from the University of Louisville School of Medicine in 2002. She completed her residency in neurology in 2006 and a fellowship in headache and facial pain with The University of North Carolina Hospitals, Chapel Hill, in 2007. Her clinical interests include treatment of migraine, face pain, occipital neuralgia, cluster headache, trigeminal neuralgia, menstrual migraine, migrainous vertigo, and other headaches as well as clinical trials in migraine, and other headaches.
She is currently an Assistant Professor of neurology with the Duke University Medical Center.

Daniel T. Laskowitz received the M.D. degree from Duke University in 1991, completed a residency in neurology at the University of Pennsylvania from 1991–1995, a fellowship in stroke and neurocritical care from 1995-1996 and a Masters of Health Science in clinical research design from Duke. He is currently a Professor and the Vice Chair of Neurology with Duke University Medical Center in Durham, NC, with cross-appointments in the Departments of Neurobiology and Anesthesiology, and serves as a Therapeutic Lead for Neurology with the Duke Clinical Research Institute. He is currently the Director of the Duke Neurovascular Laboratory. He regularly attends on the Neurosciences Intensive Care Unit. He is a fellow of the American Heart Association and American Neurological Association, and a member of the Neurocritical Care Society and American Academy of Neurology.
References
- [1].Fitzgerald G. and Hallpike C. S., “Studies in human vestibular function: I. Observations on directional preponderance (‘Nystagmusbereitschaft’) of caloric nystagmus resulting from cerebral lesions,” Brain, vol. 65, pp. 115–137, Jun. 1942. [Google Scholar]
- [2].Barany R. and Wittmaack K., “Funktionelle Prufung des Vestibularapparates (functional testing of the vestibular apparatus),” Otol. Ges., vol. 20, pp. 184–238, Oct. 1911. [Google Scholar]
- [3].Scherer H., Brandt U., Clarke A. H., Merbold U., and Parker R., “European vestibular experiments on the Spacelab-1 mission: 3. Caloric nystagmus in microgravity,” Experim. Brain Res., vol. 64, no. 2, pp. 255–263, Oct. 1986. [DOI] [PubMed] [Google Scholar]
- [4].Ma F. R., et al. , “Effects of caloric vestibular stimulation on serotoninergic system in the media vestibular nuclei of guinea pigs,” Chin. Med. J., vol. 120, no. 2, pp. 120–124, Jan. 2007. [PubMed] [Google Scholar]
- [5].Horii A., et al. , “Effect of unilateral vestibular stimulation on histamine release from the hypothalamus of rats in vivo,” J. Neurophysiol., vol. 70, no. 5, pp. 1822–1826, Nov. 1993. [DOI] [PubMed] [Google Scholar]
- [6].Horii A., Takeda N., Mochizuki T., Okakura-Mochizuki K., Yamamoto Y., and Yamatodani A., “Effects of vestibular stimulation on acetylcholine release from rat hippocampus: An in vivo microdialysis study,” J. Neurophysiol., vol. 72, no. 2, pp. 605–611, 1994. [DOI] [PubMed] [Google Scholar]
- [7].Samoudi G., Nissbrandt H., Dutia M. B., and Bergquist F., “Noisy galvanic vestibular stimulation promotes GABA release in the substantia nigra and improves locomotion in hemiparkinsonian rats,” PLoS ONE, vol. 7, no. 1, p. e29308, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yamamoto Y., Struzik Z. R., Soma R., Ohashi K., and Kwak S., “Noisy vestibular stimulation improves autonomic and motor responsiveness in central neurodegenerative disorders,” Ann. Neurol., vol. 58, no. 2, pp. 175–181, Aug. 2005. [DOI] [PubMed] [Google Scholar]
- [9].Marcelli V., et al. , “Spatio-temporal pattern of vestibular information processing after brief caloric stimulation,” Eur. J. Radiol., vol. 70, no. 2, pp. 312–316, May 2009. [DOI] [PubMed] [Google Scholar]
- [10].Grigoryan S. S., Baklavadzhyan O. G., Minasyan S. M., Adamyan T. I., Gevorkyan É. S., and Sarkisyan S. G., “Responses of hypothalamic neurons to stimulation of the vestibular nerve and lateral vestibular nucleus in the rabbit,” Neurosci. Behavioral Physiol., vol. 29, no. 1, pp. 61–66, Jan-Feb 1999. [DOI] [PubMed] [Google Scholar]
- [11].Dieterich M. and Brandt T., “The bilateral central vestibular system: Its pathways, functions, and disorders,” Ann. New York Acad. Sci., vol. 1343, pp. 10–26, Apr. 2015. [DOI] [PubMed] [Google Scholar]
- [12].Klingner C. M., et al. , “Components of vestibular cortical function,” Behavioural Brain Res., vol. 236, pp. 194–199, Sep. 2012. [DOI] [PubMed] [Google Scholar]
- [13].Dieterich M. and Brandt T., “Functional brain imaging of peripheral and central vestibular disorders,” Brain, vol. 131, no. 10, pp. 2538–2552, Oct. 2008. [DOI] [PubMed] [Google Scholar]
- [14].Adair J. C., Na D. L., Schwartz R. L., and Heilman K. M., “Caloric stimulation in neglect: Evaluation of response as a function of neglect type,” J. Int. Neuropsychol. Soc., vol. 9, pp. 983–988, Nov. 2003. [DOI] [PubMed] [Google Scholar]
- [15].Cappa S., Sterzi R., Vallar G., and Bisiach E., “Remission of hemineglect and anosognosia during vestibular stimulation,” Neuropsychologia, vol. 25, no. 5, pp. 775–782, 1987. [DOI] [PubMed] [Google Scholar]
- [16].Moon S. Y., Lee B. H., and Na D. L., “Therapeutic effects of caloric stimulation and optokinetic stimulation on hemispatial neglect,” J. Clin. Neurol., vol. 2, no. 1, pp. 12–28, Mar. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rode G., Tilikete C., Luaute J., Rossetti Y., Vighetto A., and Boisson D., “Bilateral vestibular stimulation does not improve visual hemineglect,” Neuropsychologia, vol. 40, no. 7, pp. 1104–1106, 2002. [DOI] [PubMed] [Google Scholar]
- [18].Storrie-Baker H. J., Segalowitz S. J., Black S. E., McLean J. A. G., and Sullivan N., “Improvement of hemispatial neglect with cold-water calorics: An electrophysiological test of the arousal hypothesis of neglect,” J. Int. Neuropsychol. Soc., vol. 3, pp. 394–402, Jul. 1997. [PubMed] [Google Scholar]
- [19].Sturt R. and Punt T. D., “Caloric vestibular stimulation and postural control in patients with spatial neglect following stroke,” Neuropsychol. Rehabil., vol. 23, no. 2, pp. 299–316, Apr. 2013. [DOI] [PubMed] [Google Scholar]
- [20].Vallar G., Papagno C., Rusconi M. L., and Bisiach E., “Vestibular stimulation, spatial hemineglect and dysphasia. Selective effects?” Cortex, vol. 31, no. 3, pp. 589–593, Sep. 1995. [DOI] [PubMed] [Google Scholar]
- [21].Kolev O., “How caloric vestibular irritation influences migraine attacks,” Cephalalgia, vol. 10, no. 4, pp. 167–169, Aug. 1990. [DOI] [PubMed] [Google Scholar]
- [22].McGeoch P. D., Williams L. E., Lee R. R., and Ramachandran V. S., “Behavioural evidence for vestibular stimulation as a treatment for central post-stroke pain,” J. Neurol. Neurosurgery Psychiatry, vol. 79, pp. 1298–1301, Nov. 2008. [DOI] [PubMed] [Google Scholar]
- [23].Ramachandran V. S., McGeoch P. D., Williams L., and Arcilla G., “Rapid relief of thalamic pain syndrome induced by vestibular caloric stimulation,” Neurocase, vol. 13, no. 3, pp. 185–188, Jun. 2007. [DOI] [PubMed] [Google Scholar]
- [24].Karlsen E. A., Mikhail H. H., Norris C. W., and Hassanein R. S., “Comparison of responses to air, water, and closed-loop caloric irrigators,” J. Speech Hear Res., vol. 35, pp. 186–191, Feb. 1992. [DOI] [PubMed] [Google Scholar]
- [25].Ichijo H., “Can caloric testing evaluate the function of vertical semicircular canals?” Acta Otolaryngol., vol. 131, no. 7, pp. 716–721, Jul. 2011. [DOI] [PubMed] [Google Scholar]
- [26].Maroonroge S., Emanuel D. C., and Letowski T. R., “Basic anatomy of the hearing system,” in Helmet-Mounted Displays: Sensation, Perception and Cognition Issues (279-306), Rash C. E., Russo M. B., Letowski T. R., and Schmeisser E. T., Eds. Fort Rucker, AL, USA: U.S. Army Aeromedical Research Lab, 2009. [Google Scholar]
- [27].Zangemeister W. H. and Bock O., “The influence of pneumatization of mastoid bone on caloric nystagmus response: A clinical study and a mathematical model,” Acta Otolaryngol., vol. 88, nos. 1–6, pp. 105–109, 1979. [DOI] [PubMed] [Google Scholar]
- [28].Baertschi A. J., Johnson R. N., and Hanna G. R., “A theoretical and experimental determination of vestibular dynamics in caloric stimulation,” Biol. Cybern., vol. 20, no. 3, pp. 175–186, Sep. 1975. [DOI] [PubMed] [Google Scholar]
- [29].Bock O., von Koschitzky H., and Zangemeister W. H., “Vestibular adaptation to long-term stimuli,” Biol. Cybern., vol. 33, no. 2, pp. 77–79, Jun. 1979. [DOI] [PubMed] [Google Scholar]
- [30].Bagnall M. W., McElvain L. E., Faulstich M., and du Lac S., “Frequency-independent synaptic transmission supports a linear vestibular behavior,” Neuron, vol. 60, no. 2, pp. 343–352, Oct. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Berg K., Wood-Dauphinee S., and Williams J. I., “The balance scale: Reliability assessment with elderly residents and patients with an acute stroke,” Scandin. J. Rehabil. Med., vol. 27, no. 1, pp. 27–36, Mar. 1995. [PubMed] [Google Scholar]
- [32].Heckmann J. G., Leis S., Miick-Weymann M., Hilz M. J., and Neundorfer B., “Vestibular evoked blood flow response in the basilar artery,” Acta Neurol. Scandin., vol. 100, no. 1, pp. 12–17, Jul. 1999. [DOI] [PubMed] [Google Scholar]
- [33].Serrador J. M., Schlegel T. T., Black F. O., and Wood S. J., “Vestibular effects on cerebral blood flow,” BMC Neurosci., vol. 10, p. 119, Sep. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tiecks F. P., Planck J., Haberl R. L., and Brandt T., “Reduction in posterior cerebral artery blood flow velocity during caloric vestibular stimulation,” J. Cerebral Blood Flow Metabolism, vol. 16, no. 6, pp. 1379–1382, Nov. 1996. [DOI] [PubMed] [Google Scholar]
- [35].Santina C. C. D., Potyagaylo V., Migliaccio A. A., Minor L. B., and Carey J. P., “Orientation of human semicircular canals measured by three-dimensional multiplanar CT reconstruction,” J. Assoc. Res. Otolaryngol., vol. 6, no. 3, pp. 191–206, Sep. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Spiegelberg A., Preuß M. and Kurtcuoglu V., “B-waves revisited,” Interdiscipl. Neurosurgery, vol. 6, pp. 13–17, Dec. 2016. [Google Scholar]
- [37].Newell D. W., Aaslid R., Stooss R., and Reulen H. J., “The relationship of blood flow velocity fluctuations to intracranial pressure B waves,” J. Neurosurgery, vol. 76, no. 3, pp. 415–421, Mar. 1992. [DOI] [PubMed] [Google Scholar]
- [38].Balaban C. D., Jacob R. G., and Furman J. M., “Neurologic bases for comorbidity of balance disorders, anxiety disorders and migraine: Neurotherapeutic implications,” Expert Rev. Neurotherapeutics, vol. 11, no. 3, pp. 379–394, Mar. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Buzsaki G., Rhythms of the Brain. London, U.K.: Oxford Univ. Press, 2011. [Google Scholar]
- [40].Sliwka U., Harscher S., Diehl R. R., Van Schayck R., Niesen W. D., and Weiller C., “Spontaneous oscillations in cerebral blood flow velocity give evidence of different autonomic dysfunctions in various types of headache,” Headache, vol. 41, no. 2, pp. 157–163, Feb. 2001. [PubMed] [Google Scholar]
- [41].Aurora S. K., Barrodale P. M., Tipton R. L., and Khodavirdi A., “Brainstem dysfunction in chronic migraine as evidenced by neurophysiological and positron emission tomography studies,” Headache, vol. 47, no. 7, pp. 996–1003, Jul-Aug 2007. [DOI] [PubMed] [Google Scholar]
- [42].Goadsby P. J., “Can we develop neurally acting drugs for the treatment of migraine?” Nature Rev. Drug Discovery, vol. 4, pp. 741–750, Sep. 2005. [DOI] [PubMed] [Google Scholar]
- [43].Brown J. E., Card J. P., and Yates B. J., “Polysynaptic pathways from the vestibular nuclei to the lateral mammillary nucleus of the rat: Substrates for vestibular input to head direction cells,” Exp. Brain Res., vol. 161, no. 1, pp. 47–61, Feb. 2005. [DOI] [PubMed] [Google Scholar]
- [44].Horowitz S. S., Blanchard J., and Morin L. P., “Medial vestibular connections with the hypocretin (orexin) system,” J. Comput. Neurol., vol. 487, pp. 127–146, Jun. 2005. [DOI] [PubMed] [Google Scholar]
- [45].Lipton R. B., et al. , “Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: A randomised, double-blind, parallel-group, sham-controlled trial,” Lancet Neurol., vol. 9, no. 4, pp. 373–380, Apr. 2010. [DOI] [PubMed] [Google Scholar]
- [46].Schoenen J., et al. , “Migraine prevention with a supraorbital transcutaneous stimulator: A randomized controlled trial,” Neurology, vol. 80, no. 8, pp. 697–704, Feb. 2013. [DOI] [PubMed] [Google Scholar]
- [47].Underwood E. Cadaver Study Casts Doubts on How Zapping Brain May Boost Mood, Relieve Pain. Science, Apr. 20, 2016. [Online]. Available: http://www.sciencemag.org/news/2016/04/cadaver-study-casts-doubtshow-zapping-brain-may-boost-mood-relieve-pain [Google Scholar]
- [48].Thielscher A., Antunes A., and Saturnino G. B., “Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS?” in Proc. Conf. IEEE Eng. Med. Biol. Soc., Aug. 2015, pp. 222–225. [DOI] [PubMed] [Google Scholar]
- [49].Vanzan S., Wilkinson D., Ferguson H., Pullicino P., and Sakel M., “Behavioural improvement in a minimally conscious state after caloric vestibular stimulation: Evidence from two single case studies,” Clin. Rehabil., Apr. 2016. [DOI] [PubMed]
- [50].Wilkinson D., Podlewska A., and Sakel M., “A durable gain in motor and non-motor symptoms of Parkinson’s disease following repeated caloric vestibular stimulation: A single-case study,” NeuroRehabilitation, vol. 38, no. 2, pp. 179–182, Feb. 2016. [DOI] [PubMed] [Google Scholar]
- [51].Kataoka H., et al. , “Can postural instability respond to galvanic vestibular stimulation in patients with Parkinson’s disease?” J. Movement Disorders, vol. 9, no. 1, pp. 40–43, Jan. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wuehr M., et al. , “Noisy vestibular stimulation improves dynamic walking stability in bilateral vestibulopathy,” Neurology, vol. 86, pp. 2196–2202, Jun. 2016. [DOI] [PubMed] [Google Scholar]
- [53].Wilkinson D., Ko P., Kilduff P., McGlinchey R., and Milberg W., “Improvement of a face perception deficit via subsensory galvanic vestibular stimulation,” J. Int. Neuropsychol. Soc., vol. 11, pp. 925–929, Nov. 2005. [DOI] [PubMed] [Google Scholar]
- [54].Wilkinson D., Zubko O., DeGutis J., Milberg W., and Potter J., “Improvement of a figure copying deficit during subsensory galvanic vestibular stimulation,” J. Neuropsychol., vol. 4, no. 1, pp. 107–118, Mar. 2010. [DOI] [PubMed] [Google Scholar]
- [55].Wilkinson D., Zubko O., Sakel M., Coulton S., Higgins T., and Pullicino P., “Galvanic vestibular stimulation in hemi-spatial neglec,” Frontiers Integr. Neurosci., vol. 8, p. 4, Jan. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zubko O., Wilkinson D., Langston D., and Sakel M., “The effect of repeated sessions of galvanic vestibular stimulation on target cancellation in visuo-spatial neglect: Preliminary evidence from two cases,” Brain Injury, vol. 27, no. 5, pp. 613–619, 2013. [DOI] [PubMed] [Google Scholar]
- [57].Eatock R. A. and Songer J. E., “Vestibular hair cells and afferents: Two channels for head motion signals,” Annu. Rev. Neurosci., vol. 34, pp. 501–534, Jul. 2011. [DOI] [PubMed] [Google Scholar]
- [58].Curthoys I. S. and MacDougall H. G., “What galvanic vestibular stimulation actually activates,” Frontiers Neurol., vol. 3, p. 117, Jul. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Eatock R. A., Xue J., and Kalluri R., “Ion channels in mammalian vestibular afferents may set regularity of firing,” J. Exp. Biol., vol. 211, pp. 1764–1774, Jun. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lopez C., Blanke O., and Mast F. W., “The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis,” Neuroscience, vol. 212, pp. 159–179, Jun. 2012. [DOI] [PubMed] [Google Scholar]








