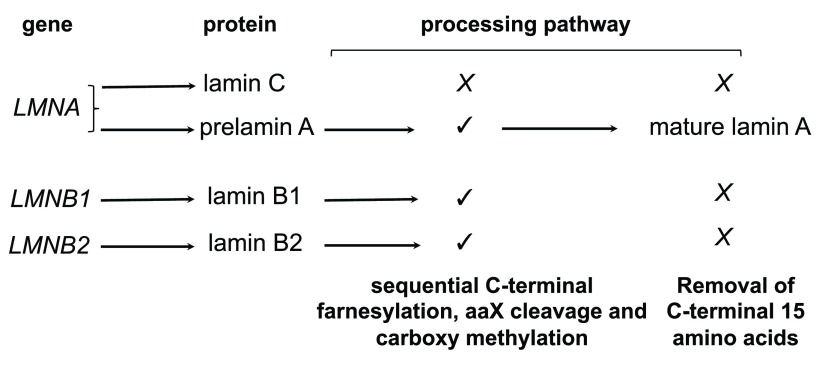
Figure 1. Major A-type and B-type lamins in mammals.
Prelamin A, lamin B1, and lamin B2 contain a carboxy-terminal CaaX motif (CSIM in human prelamin A, CAIM in lamin B1, and CYVM in lamin B2; C is cysteine, S is serine, I is isoleucine, M is methionine, A is alanine, Y is tyrosine, and V is valine) which is modified by farnesylation. This is followed by proteolysis of the aaX residues and carboxy methylation at the C-terminal end of lamin A, B1, and B2. Prelamin A undergoes further processing to remove the carboxy-terminal 15 amino acids, including the farnesylated and carboxy methylated cysteine to generate mature lamin A. In Hutchinson-Gilford progeria syndrome cells, the second cleavage site in prelamin A is deleted, and this results in the accumulation of a permanently farnesylated and carboxy methylated prelamin A variant termed progerin. Terminal cleavage of prelamin A is catalyzed by the zinc metallopeptidase ZMPSTE24, an enzyme that has recently been implicated in clearing proteins through clogged endoplasmic reticulum translocon channel 98.