Figure 6.
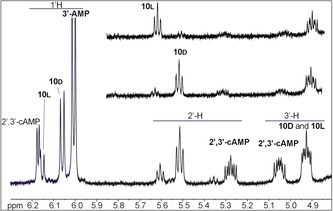
1H NMR analysis of the products of the reaction of 25 mm oxazolone 1 with 5 mm 3’‐AMP in H2O (bottom). The reaction medium was freeze‐dried and the residue dissolved in D2O. Formation of 2’,3’‐cAMP and of the diastereoisomers of the esters at the 2’‐position (identified by the comparison with similar reactions of the imidazolides obtained from the l‐form (top) and d‐form (middle) of Ac‐Tyr(Me)‐OH 4).
