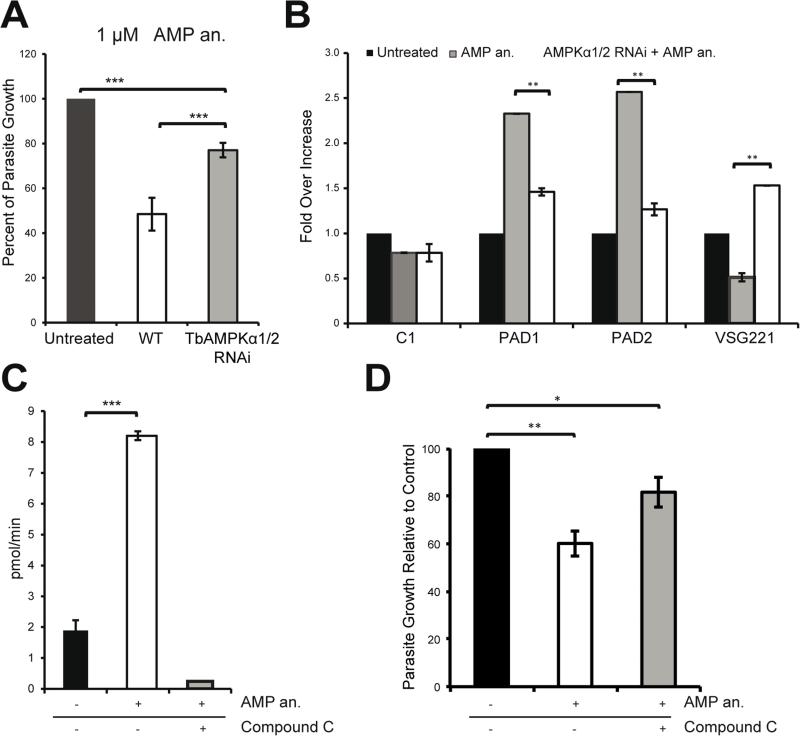
Figure 4. Cells become resistant to AMP treatment upon downregulation of the AMPKα1/2 kinases.
A. Depletion of the AMPKα1/2 subunits using RNAi counteracts the growth defect caused by AMP treatment of bloodstream cells. After 48 hours of AMPKα1/2 depletion using RNAi, T. brucei were treated for 18 hours with an AMP analog.
B. Total RNA was purified from the cells shown in (A) and the expression of the PAD1, PAD2, and VSG 221 mRNA was quantified at the transcriptional level. The regulation of these canonical differentiation genes was compromised by kinase knockdown using RNAi.
C. AMPKα1 can phosphorylate the SAMS peptide in vitro in an AMP-dependent manner. Bloodstream parasites were incubated with or without 10 μM AMP analog (8-pCPT-2’-O-Me-5’-AMP) for 30 minutes. HA-TbAMPKα1 was immunoprecipitated with an anti-HA agarose matrix. AMPK activity was assayed by 32P-ATP incorporation into the SAMS peptide. The use of compound C (CC) abolished the activation of the kinase.
D. Parasites pretreated with compound C do not show the growth defect phenotype induced by the AMP analog (8-pCPT-2’-O-Me-5’-AMP). The parasites were pretreated with 0.25 μM compound C for 4 hours and then incubated with 1 μM AMP analog for 18 hours.
All data are reported as the mean ± SEM (n=3). *, p < 0.05; **, p < 0.01; ***, p < 0.0001 using the two-tailed t-test for paired observations.