Abstract
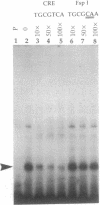
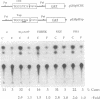
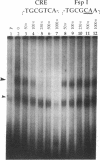
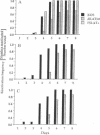
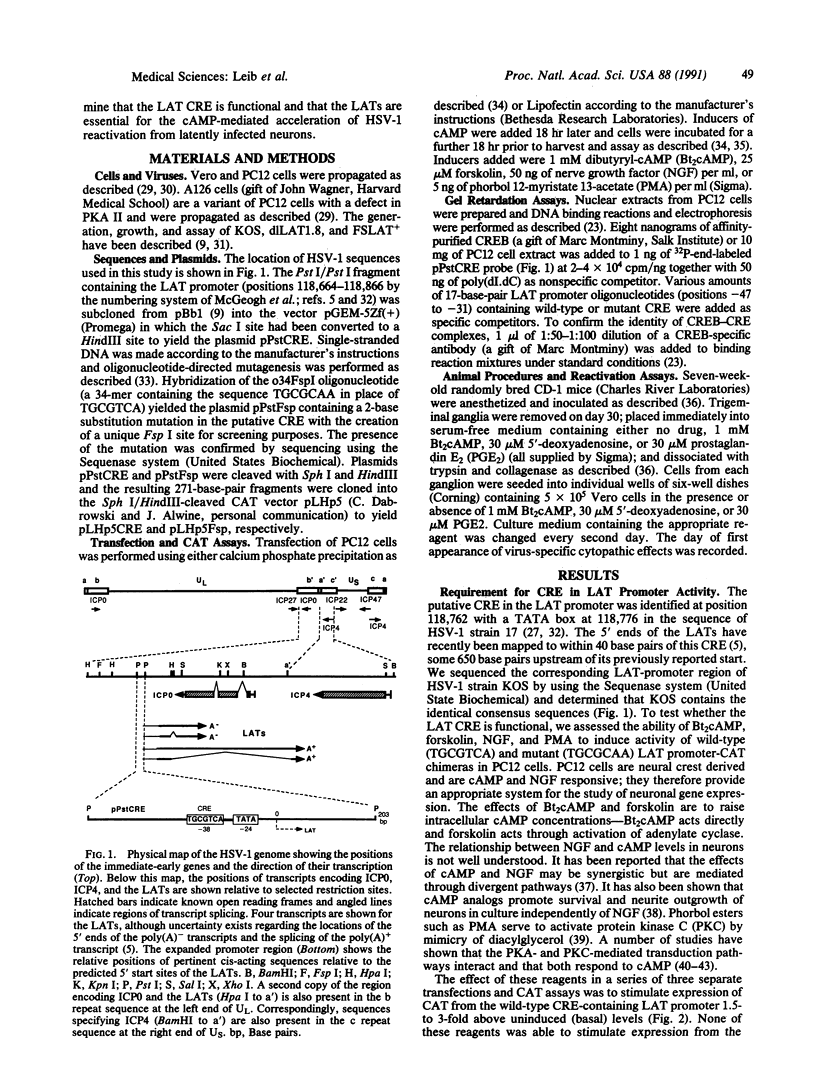
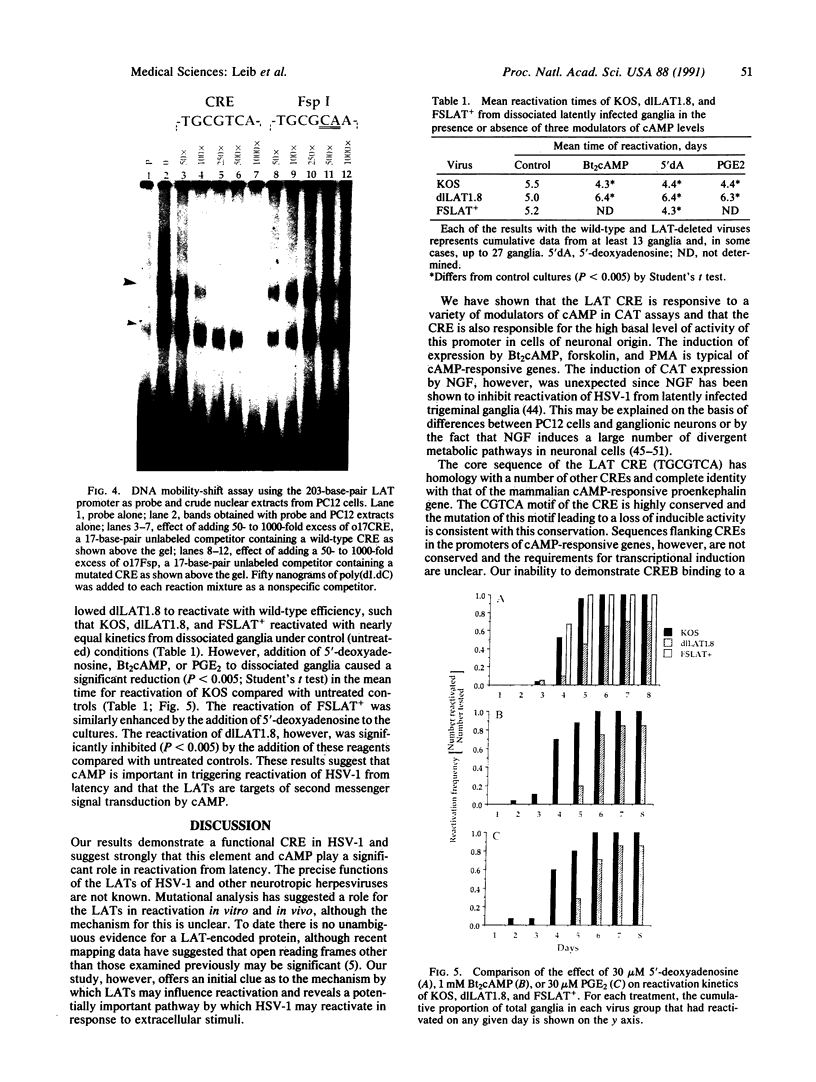
A 203-base-pair sequence 5' of the latency-associated transcripts (LATs) of herpes simplex virus type 1 contains a 7-base consensus sequence TGCGTCA that is identical to the cAMP-response element of the proenkephalin gene. This consensus sequence is at -38 relative to the putative 5' end of the LATs with a TATA box at the -24 position. In transient chloramphenicol acetyltransferase assays in rat pheochromocytoma (PC12) cells, this enhancer region stimulated gene expression up to 3-fold in the presence of dibutyryl cAMP, forskolin, nerve growth factor, or phorbol 12-myristate 13-acetate. Mutation of the cAMP-response element to TGCG-CAA resulted in a 4-fold reduction of basal activity and a complete loss of inducible stimulation. In DNA gel retardation assays, purified cAMP-response element-binding protein and a nuclear protein from PC12 cells were shown to bind specifically to this element. Furthermore, it was demonstrated that the reactivation of wild-type herpes simplex virus type 1 from dissociated latently infected murine trigeminal ganglia was significantly accelerated (P less than 0.005) by the addition of cAMP analogs or adenylate cyclase activators. However, these reagents did not accelerate reactivation of a deletion mutant that lacks the putative cAMP-response element-containing promoter region, transcriptional start site, and 1015 base pairs of the LATs. These studies demonstrate that the promoter region of the LATs contains a functional cAMP-response element and that expression of the LATs is likely controlled by second messenger signal transduction and imply a role for cAMP in triggering viral reactivation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blyth W. A., Hill T. J., Field H. J., Harbour D. A. Reactivation of herpes simplex virus infection by ultraviolet light and possible involvement of prostaglandins. J Gen Virol. 1976 Dec;33(3):547–550. doi: 10.1099/0022-1317-33-3-547. [DOI] [PubMed] [Google Scholar]
- Cambier J. C., Newell M. K., Justement L. B., McGuire J. C., Leach K. L., Chen Z. Z. Ia binding ligands and cAMP stimulate nuclear translocation of PKC in B lymphocytes. Nature. 1987 Jun 18;327(6123):629–632. doi: 10.1038/327629a0. [DOI] [PubMed] [Google Scholar]
- DeLuca N. A., Schaffer P. A. Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Mol Cell Biol. 1985 Aug;5(8):1997–2008. doi: 10.1128/mcb.5.8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A. T., Sederati F., Devi-Rao G., Flanagan W. M., Farrell M. J., Stevens J. G., Wagner E. K., Feldman L. T. Identification of the latency-associated transcript promoter by expression of rabbit beta-globin mRNA in mouse sensory nerve ganglia latently infected with a recombinant herpes simplex virus. J Virol. 1989 Sep;63(9):3844–3851. doi: 10.1128/jvi.63.9.3844-3851.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour D. A., Hill T. J., Blyth W. A. Recurrent herpes simplex in the mouse: inflammation in the skin and activation of virus in the ganglia following peripheral stimulation. J Gen Virol. 1983 Jul;64(Pt 7):1491–1498. doi: 10.1099/0022-1317-64-7-1491. [DOI] [PubMed] [Google Scholar]
- Hazel T. G., Nathans D., Lau L. F. A gene inducible by serum growth factors encodes a member of the steroid and thyroid hormone receptor superfamily. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8444–8448. doi: 10.1073/pnas.85.22.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. M., Sedarati F., Javier R. T., Wagner E. K., Stevens J. G. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology. 1990 Jan;174(1):117–125. doi: 10.1016/0042-6822(90)90060-5. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Gudas L. J. Cyclic AMP analogs and retinoic acid influence the expression of retinoic acid receptor alpha, beta, and gamma mRNAs in F9 teratocarcinoma cells. Mol Cell Biol. 1990 Jan;10(1):391–396. doi: 10.1128/mcb.10.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Jones R. H., Jones N. C. Mammalian cAMP-responsive element can activate transcription in yeast and binds a yeast factor(s) that resembles the mammalian transcription factor ANF. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2176–2180. doi: 10.1073/pnas.86.7.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara C. J., Liou H. C., Ivashkiv L. B., Glimcher L. H. A cDNA for a human cyclic AMP response element-binding protein which is distinct from CREB and expressed preferentially in brain. Mol Cell Biol. 1990 Apr;10(4):1347–1357. doi: 10.1128/mcb.10.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983 Oct 10;258(19):11442–11445. [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurane I., Tsuchiya Y., Sekizawa T., Kumagai K. Inhibition by indomethacin of in vitro reactivation of latent herpes simplex virus type 1 in murine trigeminal ganglia. J Gen Virol. 1984 Oct;65(Pt 10):1665–1674. doi: 10.1099/0022-1317-65-10-1665. [DOI] [PubMed] [Google Scholar]
- Leib D. A., Bogard C. L., Kosz-Vnenchak M., Hicks K. A., Coen D. M., Knipe D. M., Schaffer P. A. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989 Jul;63(7):2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib D. A., Coen D. M., Bogard C. L., Hicks K. A., Yager D. R., Knipe D. M., Tyler K. L., Schaffer P. A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989 Feb;63(2):759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard D. G., Gorham J. D., Cole P., Greene L. A., Ziff E. B. A nerve growth factor-regulated messenger RNA encodes a new intermediate filament protein. J Cell Biol. 1988 Jan;106(1):181–193. doi: 10.1083/jcb.106.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A. From epinephrine to cyclic AMP. Science. 1988 Aug 12;241(4867):800–806. doi: 10.1126/science.2841758. [DOI] [PubMed] [Google Scholar]
- Lokensgard J. R., Thawley D. G., Molitor T. W. Pseudorabies virus latency: restricted transcription. Arch Virol. 1990;110(1-2):129–136. doi: 10.1007/BF01310709. [DOI] [PubMed] [Google Scholar]
- Masiakowski P., Shooter E. M. Nerve growth factor induces the genes for two proteins related to a family of calcium-binding proteins in PC12 cells. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1277–1281. doi: 10.1073/pnas.85.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa H., Nirenberg M. Receptor-mediated shifts in cGMP and cAMP levels in neuroblastoma cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3472–3476. doi: 10.1073/pnas.72.9.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Mellon P. L., Clegg C. H., Correll L. A., McKnight G. S. Regulation of transcription by cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4887–4891. doi: 10.1073/pnas.86.13.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987 Nov 6;238(4828):797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988 May;1(3):183–188. doi: 10.1016/0896-6273(88)90138-9. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. Nerve growth factor rapidly induces c-fos mRNA in PC12 rat pheochromocytoma cells. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4789–4793. doi: 10.1073/pnas.83.13.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Otte A. P., van Run P., Heideveld M., van Driel R., Durston A. J. Neural induction is mediated by cross-talk between the protein kinase C and cyclic AMP pathways. Cell. 1989 Aug 25;58(4):641–648. doi: 10.1016/0092-8674(89)90099-8. [DOI] [PubMed] [Google Scholar]
- Perry L. J., McGeoch D. J. The DNA sequences of the long repeat region and adjoining parts of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Nov;69(Pt 11):2831–2846. doi: 10.1099/0022-1317-69-11-2831. [DOI] [PubMed] [Google Scholar]
- Race H. M., Wagner J. A. Nerve growth factor affects cyclic AMP metabolism, but not by directly stimulating adenylate cyclase activity. J Neurochem. 1985 May;44(5):1588–1592. doi: 10.1111/j.1471-4159.1985.tb08799.x. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Beam S. L., Mayfield J. E. Mapping bovine herpesvirus type 1 latency-related RNA in trigeminal ganglia of latently infected rabbits. J Virol. 1987 Dec;61(12):3827–3831. doi: 10.1128/jvi.61.12.3827-3831.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D. L., Hagemoser W. A., Osorio F. A., McAllister H. A. Transcription from the pseudorabies virus genome during latent infection. Brief report. Arch Virol. 1988;98(1-2):99–106. doi: 10.1007/BF01321010. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Rozengurt E., Murray M., Zachary I., Collins M. Protein kinase C activation enhances cAMP accumulation in Swiss 3T3 cells: inhibition by pertussis toxin. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2282–2286. doi: 10.1073/pnas.84.8.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydel R. E., Greene L. A. cAMP analogs promote survival and neurite outgrowth in cultures of rat sympathetic and sensory neurons independently of nerve growth factor. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1257–1261. doi: 10.1073/pnas.85.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks W. R., Greene C. C., Aschman D. P., Schaffer P. A. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985 Sep;55(3):796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz de la Maza M., Wells P. A., Foster C. S. Cyclic nucleotide modulation of herpes simplex virus latency and reactivation. Invest Ophthalmol Vis Sci. 1989 Oct;30(10):2154–2159. [PubMed] [Google Scholar]
- Schaffer P. A., Carter V. C., Timbury M. C. Collaborative complementation study of temperature-sensitive mutants of herpes simplex virus types 1 and 2. J Virol. 1978 Sep;27(3):490–504. doi: 10.1128/jvi.27.3.490-504.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I., Spivack J. G., Lirette R. P., Brown S. M., MacLean A. R., Subak-Sharpe J. H., Fraser N. W. Herpes simplex virus type 1 latency-associated transcripts are evidently not essential for latent infection. EMBO J. 1989 Feb;8(2):505–511. doi: 10.1002/j.1460-2075.1989.tb03404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Terao M., Watanabe Y., Mishina M., Numa S. Sequence requirement for transcription in vivo of the human preproenkephalin A gene. EMBO J. 1983;2(12):2223–2228. doi: 10.1002/j.1460-2075.1983.tb01727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirone F., Shooter E. M. Early gene regulation by nerve growth factor in PC12 cells: induction of an interferon-related gene. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2088–2092. doi: 10.1073/pnas.86.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk R., Corcoran T., Wagner J. A. Clonal variants of PC12 pheochromocytoma cells with defects in cAMP-dependent protein kinases induce ornithine decarboxylase in response to nerve growth factor but not to adenosine agonists. Mol Cell Biol. 1985 Aug;5(8):1984–1992. doi: 10.1128/mcb.5.8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C. L., Johnson E. M., Jr Nerve growth factor deprivation results in the reactivation of latent herpes simplex virus in vitro. J Virol. 1987 Jul;61(7):2311–2315. doi: 10.1128/jvi.61.7.2311-2315.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]