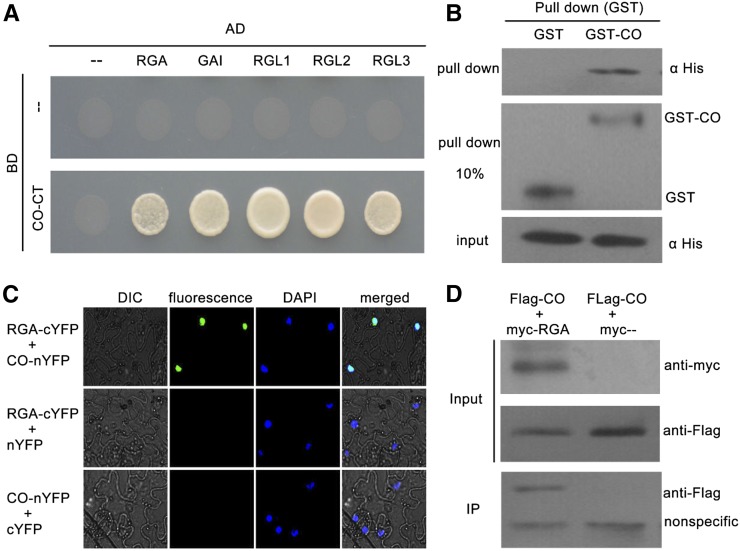
Figure 2.
Physical interactions between DELLA proteins and CO. A, Yeast two-hybrid (Y2H) analysis of DELLA-CO interactions. Interaction was indicated by the ability of cells to grow on selective media lacking Leu, Trp, His, and adenine. The Gal4 DNA binding domain (BD) and activation domain (AD) were used as negative controls. The pictures were taken 3 d after incubated at 28°C. B, In vitro GST pull-down assay for CO and RGA interaction. Soluble GST and GST-CO fusion proteins were extracted and immobilized to glutathione affinity resin. Purified GST, GST-CO were incubated with the His-RGA fusion protein from Escherichia coli cell lysate for 2 h at 4°C. The interaction was determined by western blot using anti-His antibody. The purified GST and GST-CO were diluted 10 times (pull down 10%) and detected with anti-GST antibody (middle). C, BiFC assay showing the fluorescence complementations of the cYFP fused with RGA and thenYFP fused with CO. 4’,6-Diamidino-2-phenylindole (DAPI) staining marks the nucleus. D, Co-IP assay for CO and RGA interaction. Flag-fused CO and Myc-fused RGA were transiently coexpressed in tobacco leaves. All infected leaves treated with 10 μm MG132 and 20 μm paclobutrazol for 8 h were used for Co-IP. MYC-RGA and MYC tag were immunoprecipitated with anti-MYC M2 agarose beads and detected with anti-FLAG antibodies. Protein input for Flag-CO proteins in immunoprecipitated complexes was also detected and shown.