Abstract
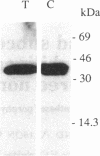
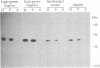
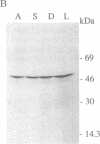
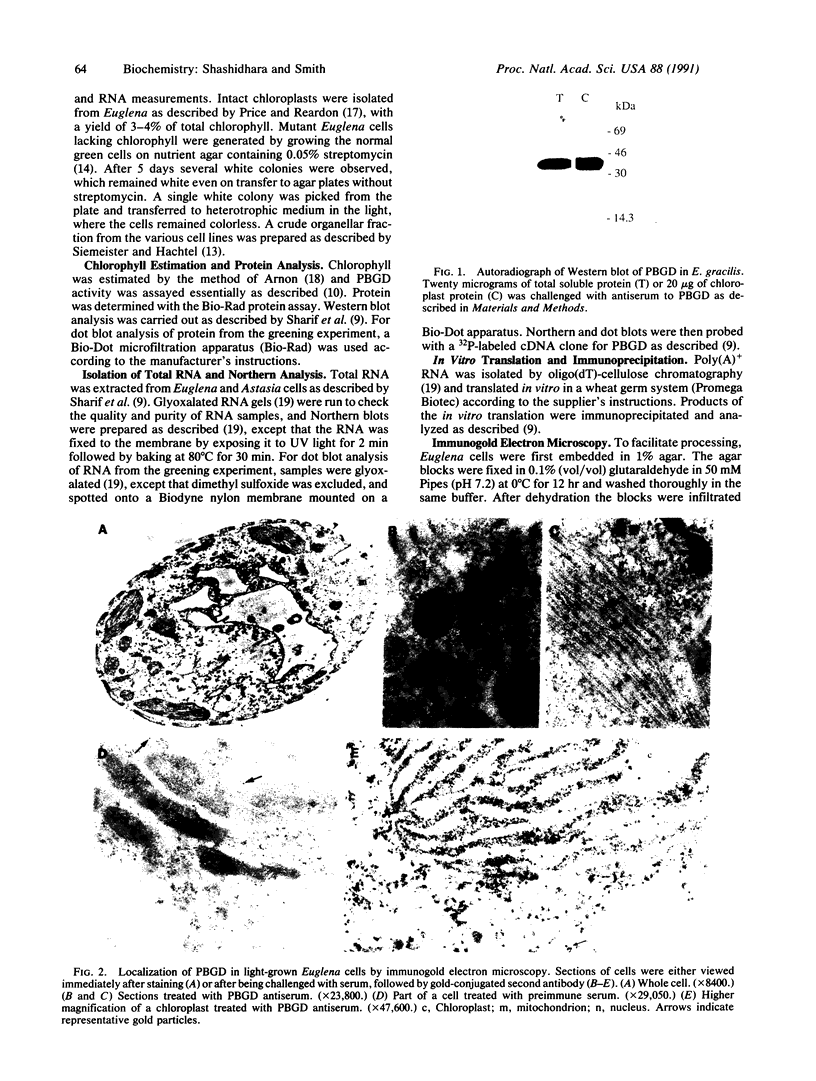
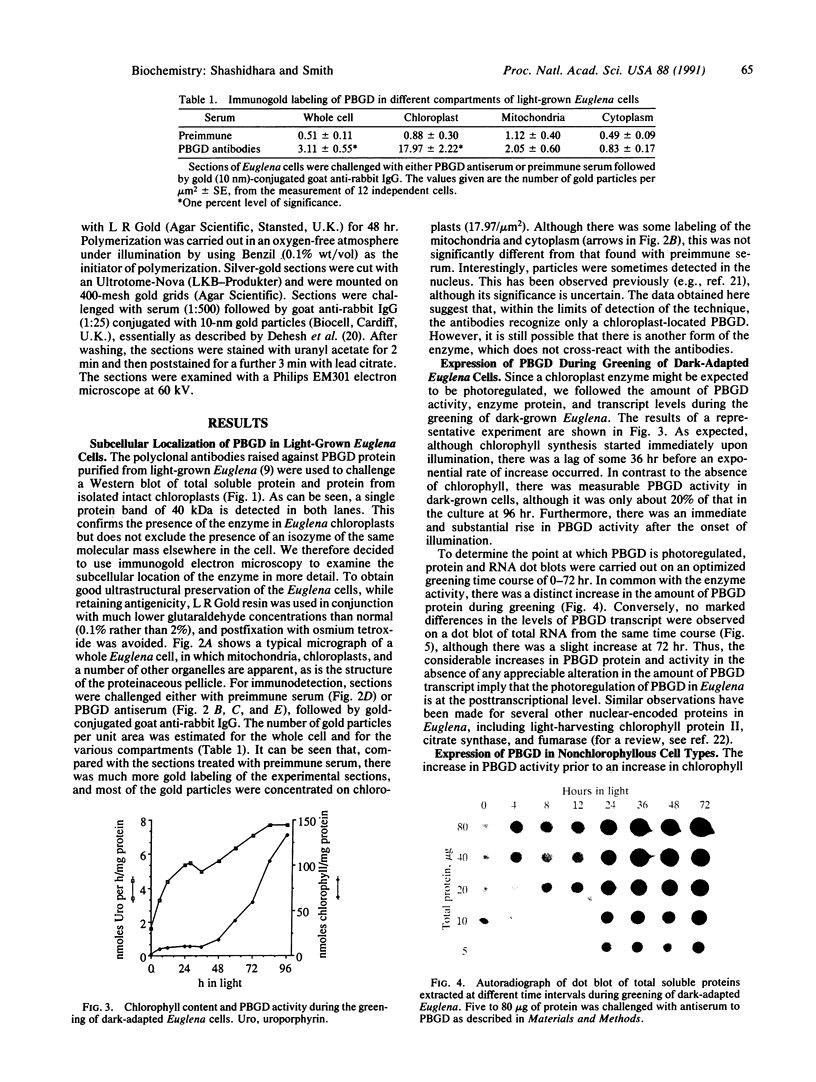
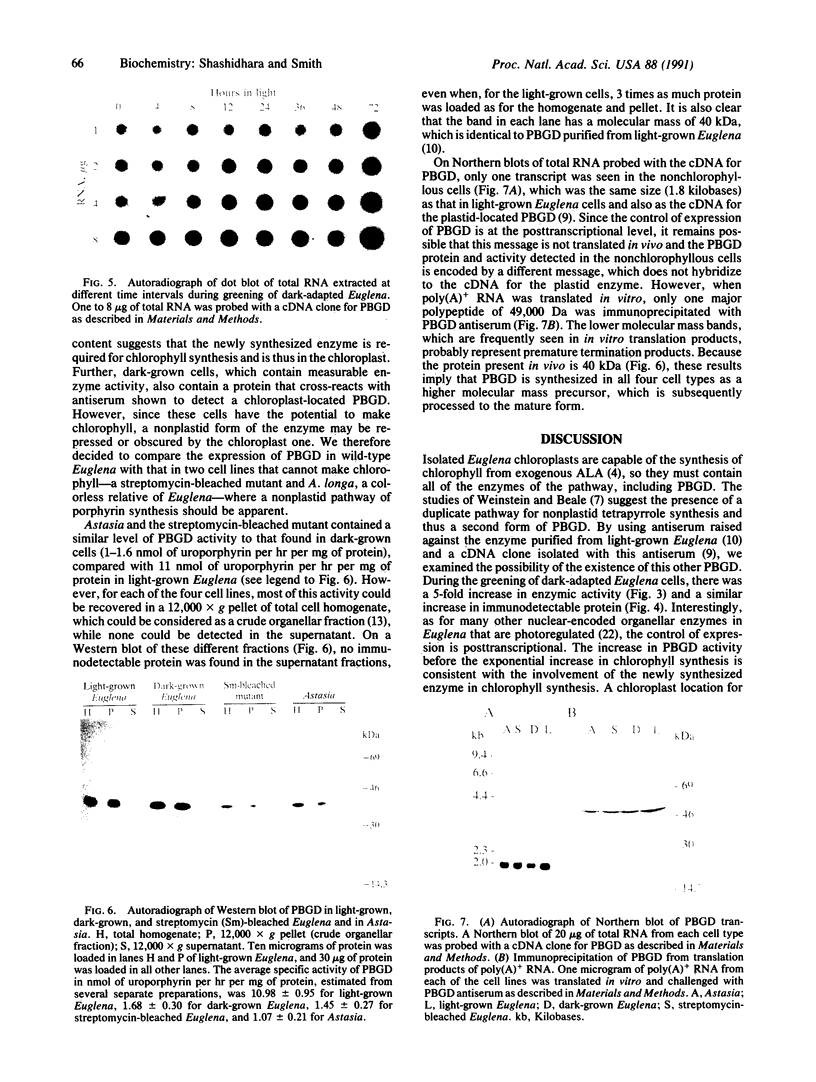
The expression and subcellular location of porphobilinogen deaminase (PBGD, also known as hydroxymethylbilane synthase; EC 4.3.1.8), one of the early enzymes of porphyrin synthesis, was investigated in light-grown Euglena and in three cell lines that do not contain chlorophyll: dark-grown Euglena, a streptomycin-bleached mutant, and Astasia longa. In wild-type Euglena, immunogold electron microscopy demonstrated that all the immunodetectable enzyme protein was in the chloroplast. PBGD was shown to be photoregulated, and like many other nuclear-encoded proteins in Euglena, the regulation was at the posttranscriptional level. In the three nonchlorophyllous cell lines, as in light-grown Euglena, a single protein of 40 kDa was detected with antiserum to PBGD. This same antiserum immunoprecipitated a larger precursor protein from the total translation products of poly(A)+ RNA, and a single transcript, which was large enough to encode the precursor, was detected on Northern blots of all four cell types. Therefore, in cells that make chlorophyll and those that do not (or cannot), PBGD is located in the plastid. No evidence was obtained for another form of the enzyme, which suggests that in Euglena there is only one pathway for the synthesis of the tetrapyrrole moiety of chlorophyll and heme.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battersby A. R., Hodgson G. L., Hunt E., McDonald E., Saunders J. Biosynthesis of porphyrins and related macrocycles. Part VI. Nature of the rearrangement process leading to the natural type III porphyrins. J Chem Soc Perkin 1. 1976;(3):273–282. doi: 10.1039/p19760000273. [DOI] [PubMed] [Google Scholar]
- Beale S. I., Foley T., Dzelzkalns V. delta-Aminolevulinic acid synthase from Euglena gracilis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1666–1669. doi: 10.1073/pnas.78.3.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovarnick J. G., Chang S. W., Schiff J. A., Schwartzbach S. D. Events surrounding the early development of Euglena chloroplasts: experiments with streptomycin in non-dividing cells. J Gen Microbiol. 1974 Jul;83(0):51–62. doi: 10.1099/00221287-83-1-51. [DOI] [PubMed] [Google Scholar]
- Ernst-Fonberg M. L., Bloch K. A chloroplast-associated fatty acid synthetase system in Euglena. Arch Biochem Biophys. 1971 Apr;143(2):392–400. doi: 10.1016/0003-9861(71)90226-8. [DOI] [PubMed] [Google Scholar]
- Foley T., Dzelzkalns V., Beale S. I. delta-Aminolevulinic Acid Synthase of Euglena gracilis: Regulation of Activity. Plant Physiol. 1982 Jul;70(1):219–226. doi: 10.1104/pp.70.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fufsler T. P., Castelfranco P. A., Wong Y. S. Formation of Mg-Containing Chlorophyll Precursors from Protoporphyrin IX, delta-Aminolevulinic Acid, and Glutamate in Isolated, Photosynthetically Competent, Developing Chloroplasts. Plant Physiol. 1984 Apr;74(4):928–933. doi: 10.1104/pp.74.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoso R., de Felipe M. R., Vivó A., Chueca A., Lázaro J. J., Gorge J. L. Immunogold localization of photosynthetic fructose-1,6-bisphosphatase in pea leaf tissue. Plant Physiol. 1989 Jan;89(1):381–385. doi: 10.1104/pp.89.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Castelfranco P. A. Regulation of 5-Aminolevulinic Acid (ALA) Synthesis in Developing Chloroplasts : III. Evidence for Functional Heterogeneity of the ALA Pool. Plant Physiol. 1990 Jan;92(1):172–178. doi: 10.1104/pp.92.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara C. G., Gough S. P., Bruyant P., Hoober J. K., Kahn A., von Wettstein D. tRNA(Glu) as a cofactor in delta-aminolevulinate biosynthesis: steps that regulate chlorophyll synthesis. Trends Biochem Sci. 1988 Apr;13(4):139–143. doi: 10.1016/0968-0004(88)90071-0. [DOI] [PubMed] [Google Scholar]
- Lützow M., Kleinig H. Chlorophyll-free chromoplasts from daffodil contain most of the enzymes for chlorophyll synthesis in a highly active form. Arch Biochem Biophys. 1990 Feb 15;277(1):94–100. doi: 10.1016/0003-9861(90)90555-d. [DOI] [PubMed] [Google Scholar]
- Sharif A. L., Smith A. G., Abell C. Isolation and characterisation of a cDNA clone for a chlorophyll synthesis enzyme from Euglena gracilis. The chloroplast enzyme hydroxymethylbilane synthase (porphobilinogen deaminase) is synthesised with a very long transit peptide in Euglena. Eur J Biochem. 1989 Sep 15;184(2):353–359. doi: 10.1111/j.1432-1033.1989.tb15026.x. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Separate physiological roles and subcellular compartments for two tetrapyrrole biosynthetic pathways in Euglena gracilis. J Biol Chem. 1983 Jun 10;258(11):6799–6807. [PubMed] [Google Scholar]