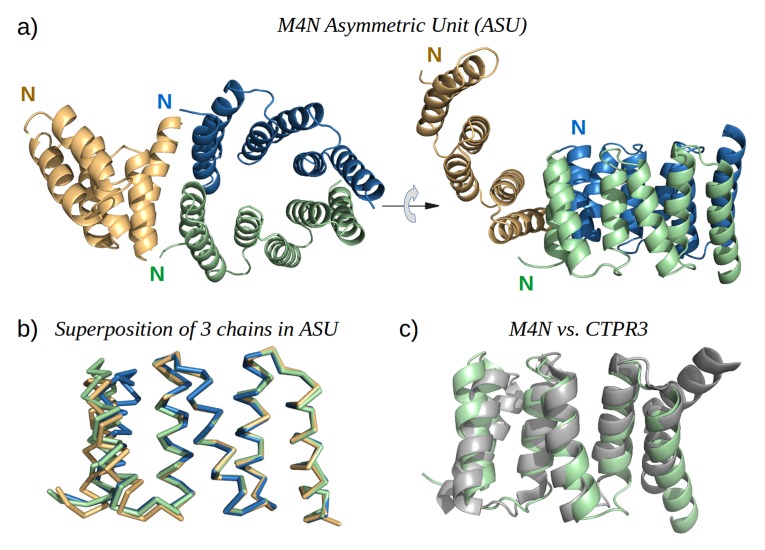
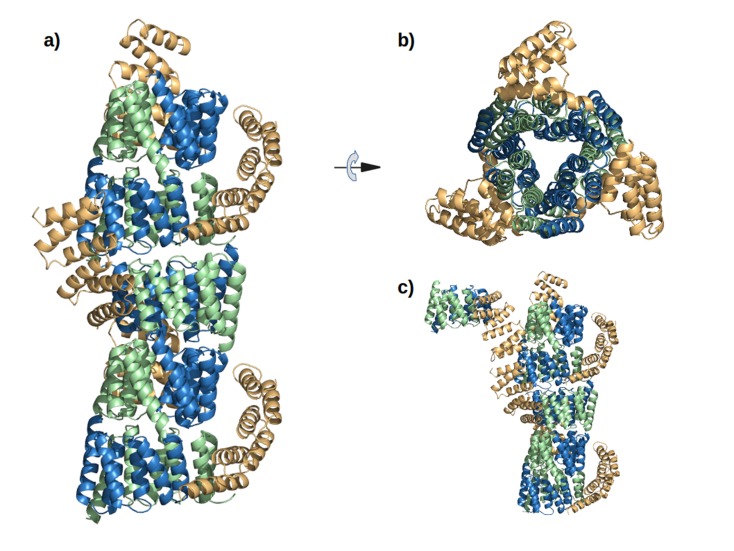
Figure 5. The X-ray structure of M4N.
(a) The three chains A, B and C in the asymmetric unit are colored green, blue and yellow, respectively. Chains A and B form a dimer. (b) Superposition of the three chains. Only Cα traces are shown for clarity. (c) Superposition of M4N (chain A, green) and the designed consensus TPR CTPR3 (PDB: 1na0, chain A, gray).