Abstract
Background
Emergency endoscopy for every patient with upper gastrointestinal hemorrhage is not possible in many medical centers. Simple guidelines to select patients for emergency endoscopy are lacking. The aim of the present report is to develop a simple scoring system to classify upper gastrointestinal hemorrhage (UGIH) severity based on patient clinical profiles at the emergency departments.
Methods
Retrospective data of patients with UGIH in a university affiliated hospital were analyzed. Patients were criterion-classified into 3 severity levels: mild, moderate and severe. Clinical and laboratory information were compared among the 3 groups. Significant parameters were selected as indicators of severity. Coefficients of significant multivariable parameters were transformed into item scores, which added up as individual severity scores. The scores were used to classify patients into 3 urgency levels: non-urgent, urgent and emergent groups. Score-classification and criterion-classification were compared.
Results
Significant parameters in the model were age ≥ 60 years, pulse rate ≥ 100/min, systolic blood pressure < 100 mmHg, hemoglobin < 10 g/dL, blood urea nitrogen ≥ 35 mg/dL, presence of cirrhosis and hepatic failure. The score ranged from 0 to 27, and classifying patients into 3 urgency groups: non-urgent (score < 4, n = 215, 21.2%), urgent (score 4 - 16, n = 677, 66.9%) and emergent (score > 16, n = 121, 11.9%). The score correctly classified 81.4% of the patients into their original (criterion-classified) severity groups. Under-estimation (7.5%) and over-estimation (11.1%) were clinically acceptable.
Conclusions
Our UGIH severity scoring system classified patients into 3 urgency groups: non-urgent, urgent and emergent, with clinically acceptable small number of under- and over-estimations. Its discriminative ability and precision should be validated before adopting into clinical practice.
Keywords: Gastrointestinal hemorrhage, Gastrointestinal bleeding, Gastroscopy, Prognostic indicators, Scoring system, Clinical prediction rules
Introduction
Upper gastrointestinal hemorrhage (UGIH) is a common challenge encountered in emergency medicine departments. Hospital admissions were approximately 300,000 cases per year in the United States [1]. Case fatalities were also as high as 7-10%, with a yearly expenses of 2.5 billion $US [2].
Endoscopy plays a key role in classifying patients. It is generally suggested that endoscopy should be scheduled within 24 hours after hospital admission [3-6]. The American Society for Gastrointestinal Endoscopy suggested a somewhat earlier timing, within 12 hours [7], but actual early endoscopic examinations were usually scheduled between 2 to 24 hours [8-13]. Almost 80% of UGIH is self-limited [14]. Therefore in most cases endoscopy could be postponed to the following day. This implies that there are only a fraction of patients who actually required an emergency endoscopy. However, this is possible only in hospitals which are 24-hour well-equipped [15]. Clinicians all agreed that endoscopy for patients with mild and moderate UGIH could practically be delayed, and that emergency endoscopy is necessary only for some patients with severe bleeding or those who are in a state of shock. Existing screening procedures for patients with UGIH were mostly based on a scoring system that stratifies patients into those with high or low risk, focusing on ultimate clinical outcomes such as re-bleeding and/or death [16-18]. The purpose of such screening was mainly to help clinicians discharge patients with low risk early and safely, to be treated as out-patients, and to selectively admit patients with high risk to an intensive care unit for close monitoring [16-18].
Our study focused on developing a simple scoring system to predict UGIH severity, by using patient clinical profiles on arrival at the emergency departments, as had been reported earlier [19]. The scores may be used to identify and discriminate UGIH patients with different severity levels without depending entirely on endoscopic examinations and findings.
Materials and Methods
Patients and methods
The study was conducted in Kamphaeng Phet Hospital, a university-affiliated tertiary hospital in the northern region of Thailand. We retrieved medical files of patients who presented to the emergency department with upper gastrointestinal bleeding, between 2009 and 2010. The ICD10 keywords for hospital database search were: K920-Hematemesis, K921-Melena and K922-Gastrointestinal hemorrhage unspecified.
Indicator parameters
The patient profiles of interest were: 1) Demographic profiles: gender and age; 2) Mode of presentation: hematemesis, coffee ground vomiting, hematochezia, melena, and syncope; 3) Hemodynamic profiles: pulse rate and systolic blood pressure; 4) Biochemical profiles: hemoglobin and blood urea nitrogen, and 5) Co-morbidities: presence of cirrhosis, hepatic failure, cardiac failure and renal failure.
Definitions of UGIH severity: an outcome of interest
We used the following criteria to define UGIH severity: 1) Severe: patients who required surgical interventions to stop bleeding, patients in a state of grade 3 and 4 shock [20], and patients who did not survive; 2) Moderate: patients who required endoscopy to stop bleeding (endotherapy), patients with re-bleeding, patients in a state of grade 1 and 2 shock, and patients who required blood transfusion; 3) Mild: patients with no signs of shock, patients who required endoscopy without hemostasis, and patients who did not required any blood transfusions.
Data analysis
The patient profiles were compared across the three severity groups by chi-squared tests for linear trends, or two-way ANOVA by rank. Significant indicators for UGIH severity were presented by odds ratios from a multivariable ordinal continuation ratio logistic regression, which is most suitable for ordinal-natured outcomes such as in this study. The significant coefficients were transformed into item scores and added up to a single total score for each patient. The discriminative performances of the scores were displayed graphically. The patients were classified by the scores into 3 urgency groups corresponding to their original severity: non-urgent, urgent and emergent. The score-classification of urgency was compared to the criterion-classification of severity.
The study was approved by The Kamphaeng Phet Hospital Ethical Committee for Clinical Research. No patients inform consents were required for this retrospective data collection. Traceable patient information was omitted in all processes of data analysis and presentations. The authors declared no out-source grants received and no conflicts of interests.
Results
We retrieved 1,043 medical files corresponding with the above definitions. Among these, 255 were criterion-classified as mild, 664 as moderate, and 124 as severe.
Significant predictors
By a univariable analysis, patients in the 3 severity groups were similar according to gender (P = 0.871), presence of hematemesis (P = 0.315), hematochezia (P = 0.462), and hepatic failure (P = 0.071), but were different according to age, coffee ground vomiting, melena, syncope, pulse rate, systolic blood pressure, hemoglobin, blood urea nitrogen, presence of cirrhosis, cardiac failure, and renal failure (Table 1).
Table 1. Characteristics of Patients With Upper Gastrointestinal Hemorrhage, Criterion-Classified into Three Severity Levels.
| Patient characteristics | Mild | Moderate | Severe | P-value* |
|---|---|---|---|---|
| n = 255 mean ± SD |
n = 664 mean ± SD |
n = 124 mean ± SD |
||
| Demographics | ||||
| Male (n, %) | 175 (68.6) | 427 (64.3) | 87 (70.2) | 0.871 |
| Age (year) | 54.6 ± 18.0 | 60.4 ± 14.8 | 58.4 ± 14.2 | 0.010 |
| Mode of presentation (n, %) | ||||
| Hematemesis | 117 (45.9) | 299 (45.0) | 66 (53.2) | 0.315 |
| Coffee ground vomiting | 67 (26.3) | 114 (17.2) | 26 (21.0) | 0.048 |
| Hematochezia | 20 (7.8) | 40 (6.0) | 8 (6.5) | 0.462 |
| Melena | 113 (44.3) | 421 (63.4) | 69 (55.7) | 0.001 |
| Syncope | 28 (11.0) | 144 (21.7) | 34 (27.4) | < 0.001 |
| Hemodynamics | ||||
| Pulse (/min) | 89.8 ± 16.3 | 91.4 ± 15.7 | 93.1 ± 17.7 | 0.022 |
| SBP (mmHg) | 128.6 ± 21.6 | 120.6 ± 20.5 | 88.5 ± 17.0 | < 0.001 |
| Biochemicals | ||||
| Hemoglobin (g/dL) | 11.4 ± 2.4 | 7.0 ± 2.1 | 7.4 ± 2.9 | < 0.001 |
| BUN (mg/dL) | 23.9 ± 16.5 | 36.5 ± 21.7 | 37.6 ± 22.5 | < 0.001 |
| Co-morbidities (n, %) | ||||
| Cirrhosis | 14 (5.5) | 106 (16.0) | 28 (22.6) | < 0.001 |
| Hepatic failure | 0 (0) | 6 (0.9) | 2 (1.6) | 0.071 |
| Cardiac failure | 1 (0.4) | 6 (0.9) | 4 (3.2) | 0.024 |
| Renal failure | 4 (1.6) | 53 (8.0) | 12 (9.7) | < 0.001 |
| Clinical outcomes (n, %) | ||||
| Re-bleeding | 6 (2.4) | 42 (6.3) | 18 (14.5) | < 0.001 |
| Dead | 0 (0) | 1 (0.2) | 24 (19.4) | < 0.001 |
*P-value from chi-squared for linear trends, or two-way ANOVA by rank. SD: standard deviation; SBP: systolic blood pressure; BUN: blood urea nitrogen.
Under a multivariable analysis, the remaining patient profiles that significantly increased UGIH severity levels were: age ≥ 60 years (OR = 1.57, 95% CI = 1.13 - 2.18, P = 0.007), pulse rate ≥ 100/min (OR = 1.56, 95% CI = 1.11 - 2.19, P = 0.011), systolic blood pressure < 100 mmHg (OR = 97.49, 95% CI = 54.86 - 173.25, P < 0.001), hemoglobin < 10 g/dL (OR = 15.00, 95% CI = 10.48 - 21.46, P < 0.001), blood urea nitrogen ≥ 35 mg/dL (OR = 2.22, 95% CI = 1.57 - 3.14, P < 0.001), presence of cirrhosis (OR = 2.55, 95% CI = 1.58 - 4.14, P < 0.001) and presence of hepatic failure (OR = 8.12, 95% CI = 1.66 - 39.67, P = 0.010). The first two strongest predictors were systolic blood pressure < 100 mmHg (OR = 97.49) and hemoglobin < 10 g/dL (OR = 15.00) (Table 2).
Table 2. Significant Predictors of Upper Gastrointestinal Hemorrhage Severity and Assigned Item Score.
| Predictors | Category | OR | 95% CI | P-value | Coefficient* | Score |
|---|---|---|---|---|---|---|
| Age (year) | ≥ 60 | 1.57 | 1.13 - 2.18 | 0.007 | 0.45 | 1 |
| < 60 | 1.00 | ref | 0 | |||
| Pulse (/min) | ≥ 100 | 1.56 | 1.11 - 2.19 | 0.011 | 0.44 | 1 |
| < 100 | 1.00 | ref | 0 | |||
| Systolic pressure (mmHg) | < 100 | 97.49 | 54.86 - 173.25 | < 0.001 | 4.58 | 10.5 |
| ≥ 100 | 1.00 | ref | 0 | |||
| Hemoglobin (g/dL) | < 10 | 15.00 | 10.48 - 21.46 | < 0.001 | 2.71 | 6 |
| ≥ 10 | 1.00 | ref | 0 | |||
| BUN (mg/dL) | ≥ 35 | 2.22 | 1.57 - 3.14 | < 0.001 | 0.80 | 2 |
| < 35 | 1.00 | Ref | 0 | |||
| Cirrhosis | yes | 2.55 | 1.58 - 4.14 | < 0.001 | 0.94 | 2 |
| no | 1.00 | Ref | 0 | |||
| Hepatic failure | yes | 8.12 | 1.66 - 39.67 | 0.010 | 2.09 | 4.5 |
| no | 1.00 | ref | 0 |
*Coefficients from multivariable continuation ratio logistic regression. OR: odds ratio; CI: confidence interval; ref: reference category; BUN: blood urea nitrogen.
The scoring system
The above significant coefficients were divided by the smallest coefficient (0.44) and rounded up or down to the nearest 0.5 integers to serve as item scores. Scores were not available for 30 patients with missing information of key variables. The item scores ranged from 0 to 10.5 and the sum (total) scores may range from 0 to 27 (Table 2).
Discriminations
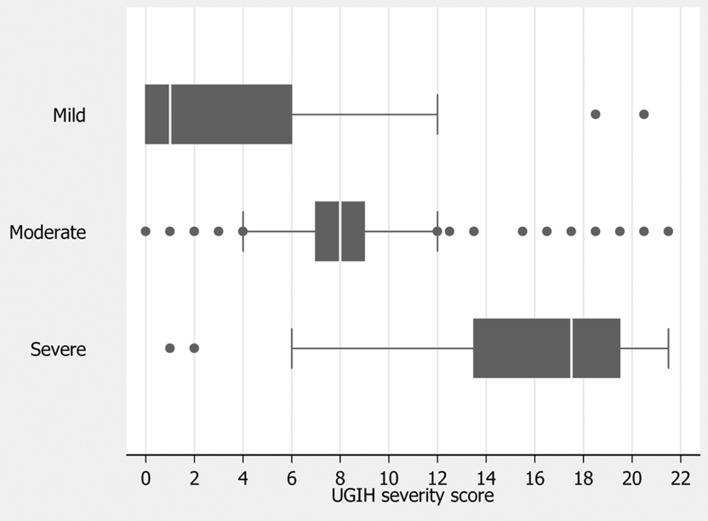
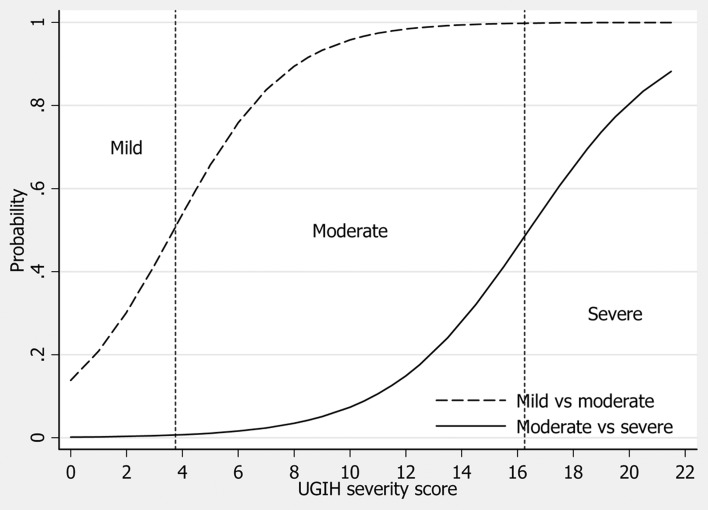
The mean scores for patients in the mild, moderate and severe groups were 3.0 ± 3.5, 8.3 ± 3.3, and 16.1 ± 4.2 respectively (Table 3), and clustered within different distributions (Fig. 1). The derived scores discriminated moderate UGIH from mild UGIH at moderate scores, and discriminated severe UGIH from moderate UGIH at higher scores (Fig. 2).
Table 3. Score-Classified Urgency Levels, Criterion-Classified Severity Levels, and Risk Estimation Validity.
| Score-classified urgency levels | Score range | Criterion-classified severity levels | Risk estimation validity* | |||||
|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Over | Correct | Under | |||
| n = 247 | n = 650 | n = 116 | (%) | (%) | (%) | |||
| Mean ± SD | 3.0 ± 3.5 | 8.3 ± 3.3 | 16.1 ± 4.2 | |||||
| IQR** | 0 - 6 | 7-9 | 13.5-19.5 | |||||
| Non-urgent | n = 215 | < 4 | 174 | 39 | 2 | - | 17.2 | 4.0 |
| Urgent | n = 677 | 4 - 16 | 71 | 571 | 35 | 7.0 | 56.4 | 3.5 |
| Emergent | n = 121 | > 16 | 2 | 40 | 79 | 4.1 | 7.8 | - |
| Total | 11.1 | 81.4 | 7.5 | |||||
*Percentage of total patients. SD: standard deviation; IQR: Inter-quartile range.
Figure 1.
Distribution of UGIH severity scores by criterion-classified severity levels. Vertical lines in box represent medians. Box boundaries represent 25th and 75th percentiles.
Figure 2.
Discrimination of urgency based on UGIH severity scores. Dash line: mild (non-urgent) vs. moderate (urgent). Solid line: moderate (urgent) vs. severe (emergent). Vertical dotted lines represent boundaries (cut-off points) of the scores.
Calibrations
For each of the 3 levels of severity, the score-predicted percents were calibrated against the criterion-classified percents. The compared percentages agreed correspondingly, yielding P-values (goodness-of-fit statistics) of 0.992, 0.996 and 0.992 for prediction of mild, moderate and severe UGIH respectively, implying no lack-of-fits.
Clinical predictions
The score predicted patients who were at least in a moderate group (moderate or severe vs. mild) with high percentage (area under the ROC curve = 0.8813; 95% CI = 0.8600 - 0.9008, data not shown), and predict patients who were in a severe (severe vs. mild or moderate) group with greater proportion (area under the ROC = 0.9274; 95% CI = 0.9092 - 0.9422, data not shown).
For clinical purposes, patients were score-classified into 3 groups: scores < 4, non-urgent; scores 4 - 16, urgent; and scores > 16, emergent. The scores of < 4 correctly classified 174 out of 247 “mild” patients as “non-urgent”, with 1-level under-estimation in 39 patients and 2-level under-estimation in 2 patients (an overall under-estimation of 4.0%). A score of 4 - 16 correctly classified 571 out of 650 “moderate” patients as “urgent”, with 35 cases (3.5%) under-estimation and 71 cases (7.0%) over-estimation. A score of > 16 classified 79 out of 116 “severe” patients as “emergent”, with 40 patients 1-level over-estimation, and 2 patients 2-level over-estimation (an overall over-estimation of 4.1%) (Table 3).
Discussion
The development of scoring systems for screening patients presenting with UGIH may be classified into 2 groups. The first group used information on clinical and laboratory parameters without endoscopic findings. Examples are the Blatchford Score [21], the Bleed Risk Classification [22, 23], and the Clinical Rockall Score. The second group used endoscopic examinations in addition to clinical and laboratory parameters. Examples are the Complete Rockall Score, the Baylor-College Score [24] and the Cedars-Sinai Score [25]. Advantages or disadvantages of the two scoring systems depend on the patient settings and clinical outcomes to be predicted. The Blatchford Score has high sensitivity in predicting the need for intervention, but its low specificity results in obvious overestimation [26]. The Rockall Score has a good prediction for death, but a poor prediction for re-bleeding, or the need for surgical procedures [27].
The existing scoring systems to classify UGIH patients into severity levels, like the Blatchford Score [21] or the Rockall Score, classified patients into only 2 groups, high risk and low risk. Very narrow ranges of the scores seemed to cause some limitations on clinical practice. In the Blatchford Score, patients scoring 0 were classified as the “low risk” [21, 28], and those scoring 1 or more as the “high risk”. The same rule was also used in the Rockall Score [28, 29]. As there are only a small number of patients in the low risk group, the score classified a large number of patients as “high risk”. For examples, using the Blatchford Score, there would be 92.1% of patients classified as the “high risk”, 81.6% from using the Clinical Rockall Score, and 70.1% by using the Complete Rockall Score [28]. This over classification will results in increasing patient loads and medical resources overuse.
Classifying patients into broader ranges may be more practical for clinicians and surgeons. Our study used 2 cut-off points to classify patients into 3 groups: non-urgent, urgent and emergent. Directive actions are correspondingly suggested as follows: 1) Patients scoring < 4, the “non-urgent” representing “mild” group, had low level of severity. They could be managed conservatively. No blood transfusion may be needed, and elective endoscopy may be appointed in 96 hours to 10 days. These patients may be treated as out-patients. This low risk group correspond to the “low risk” scoring 0 in the Blatchford Score and the Pre-Endoscopic Rockall Score, in which patients were successfully treated as out-patients, the re-bleeding rate was very low and no deaths reported [5, 22]; 2) Patients scoring between 4 and 16, the “urgent” representing the “moderate” group, had a higher severity level. These patients should be admitted to hospital with additional interventions. Resuscitation may be needed. Fluid replacement and/or blood transfusion may be given as indicated. Endoscopy should be appointed within 24 - 96 hours after admission. Patients in this group comprised approximately 60% of all UGIH patients and corresponded to some patients in the high risk group from the Blatchford Score and the Rockall Score [5, 22]; 3) Patients scoring > 16, the “emergent” representing the “severe” group, had the highest severity level. These patients may experience blood pressure drop. Vigorous resuscitation may be needed. They should be admitted to an intensive care unit (ICU) for close monitoring. Endoscopy should be appointed immediately, within 24 hours, or as soon as vital signs are stable. Some patients may require surgical interventions to stop bleeding or for life savings. These patients corresponded to the “high risk” group in both the Blatchford Score and the Rockall Score.
Our algorithm classified 21.2% patients as the mild group, similar percentages to the Blatchford Score and the Rockall Score [22], but the number of patients in the high risk group would be diminished from 75-80% by the Blatchford Score [30] and the Rockall Score to only 11.9%. The rest of the patients would be classified as “moderate” or “urgent” group instead (66.9%). This algorithm will cut down the number of patients requiring immediate endoscopy (< 24 hours) to those who were actually severe. The unnecessary cost of care, both medical personnel, medical instruments, and other medical resources for mild and moderate patients could vastly be reduced.
Our scoring system relied on similar clinical and laboratory parameters, without using endoscopic findings, as the Blatchford Score [21] and the Clinical Rockall Score [5]. It should therefore be usable in many settings where endoscopy is unavailable. Primary or secondary care settings may use this scoring system to selectively transfer “severe” patients to the nearest tertiary care settings. Like other scoring systems developed, our score also need external validation to confirm its discriminative ability and precision.
Conclusions
Patient profiles might be combined to develop a simple UGIH severity scoring system, which could classify patients into non-urgent, urgent and emergent groups. Small numbers of under- and over-estimations were clinically acceptable. However, its discriminative ability and precision should be validated with a new group of similar patients.
Acknowledgments
The authors wish to thank Kamchai Rangsimanpaiboon, the director of Kamphaeng Phet Hospital for his strong support.
Ethical Approval
The study was approved by The Kamphaeng Phet Hospital Ethical Committee for Clinical Research.
Conflicts of Interests
None declared.
References
- 1.Yavorski RT, Wong RK, Maydonovitch C, Battin LS, Furnia A, Amundson DE. Analysis of 3,294 cases of upper gastrointestinal bleeding in military medical facilities. Am J Gastroenterol. 1995;90(4):568–573. [PubMed] [Google Scholar]
- 2.Gilbert DA. Epidemiology of upper gastrointestinal bleeding. Gastrointest Endosc. 1990;36(5 Suppl):S8–13. [PubMed] [Google Scholar]
- 3.Barkun A, Bardou M, Marshall JK. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139(10):843–857. doi: 10.7326/0003-4819-139-10-200311180-00012. [DOI] [PubMed] [Google Scholar]
- 4.Adler DG, Leighton JA, Davila RE, Hirota WK, Jacobson BC, Qureshi WA, Rajan E. et al. ASGE guideline: The role of endoscopy in acute non-variceal upper-GI hemorrhage. Gastrointest Endosc. 2004;60(4):497–504. doi: 10.1016/S0016-5107(04)01568-8. [DOI] [PubMed] [Google Scholar]
- 5.Rockall TA, Logan RF, Devlin HB, Northfield TC. Selection of patients for early discharge or outpatient care after acute upper gastrointestinal haemorrhage. National Audit of Acute Upper Gastrointestinal Haemorrhage. Lancet. 1996;347(9009):1138–1140. doi: 10.1016/S0140-6736(96)90607-8. [DOI] [PubMed] [Google Scholar]
- 6.Eisen GM, Dominitz JA, Faigel DO, Goldstein JL, Kalloo AN, Petersen BT, Raddawi HM. et al. An annotated algorithmic approach to upper gastrointestinal bleeding. Gastrointest Endosc. 2001;53(7):853–858. doi: 10.1016/s0016-5107(01)70305-7. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert DA, Silverstein FE, Tedesco FJ, Buenger NK, Persing J. The national ASGE survey on upper gastrointestinal bleeding. III. Endoscopy in upper gastrointestinal bleeding. Gastrointest Endosc. 1981;27(2):94–102. doi: 10.1016/S0016-5107(81)73157-2. [DOI] [PubMed] [Google Scholar]
- 8.Hsu PI, Lin XZ, Chan SH, Lin CY, Chang TT, Shin JS, Hsu LY. et al. Bleeding peptic ulcer—risk factors for rebleeding and sequential changes in endoscopic findings. Gut. 1994;35(6):746–749. doi: 10.1136/gut.35.6.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cipolletta L, Bianco MA, Rotondano G, Marmo R, Piscopo R. Outpatient management for low-risk nonvariceal upper GI bleeding: a randomized controlled trial. Gastrointest Endosc. 2002;55(1):1–5. doi: 10.1067/mge.2002.119219. [DOI] [PubMed] [Google Scholar]
- 10.Lin HJ, Wang K, Perng CL, Chua RT, Lee FY, Lee CH, Lee SD. Early or delayed endoscopy for patients with peptic ulcer bleeding. A prospective randomized study. J Clin Gastroenterol. 1996;22(4):267–271. doi: 10.1097/00004836-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Lee JG, Turnipseed S, Romano PS, Vigil H, Azari R, Melnikoff N, Hsu R. et al. Endoscopy-based triage significantly reduces hospitalization rates and costs of treating upper GI bleeding: a randomized controlled trial. Gastrointest Endosc. 1999;50(6):755–761. doi: 10.1016/S0016-5107(99)70154-9. [DOI] [PubMed] [Google Scholar]
- 12.Cooper GS, Chak A, Connors AF Jr, Harper DL, Rosenthal GE. The effectiveness of early endoscopy for upper gastrointestinal hemorrhage: a community-based analysis. Med Care. 1998;36(4):462–474. doi: 10.1097/00005650-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Chak A, Cooper GS, Lloyd LE, Kolz CS, Barnhart BA, Wong RC. Effectiveness of endoscopy in patients admitted to the intensive care unit with upper GI hemorrhage. Gastrointest Endosc. 2001;53(1):6–13. doi: 10.1067/mge.2001.108965. [DOI] [PubMed] [Google Scholar]
- 14.British Society of Gastroenterology Endoscopy Committee. Non-variceal upper gastrointestinal haemorrhage: guidelines. Gut. 2002;51(Suppl 4):iv1–iv6. doi: 10.1136/gut.51.suppl_4.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corley DA, Stefan AM, Wolf M, Cook EF, Lee TH. Early indicators of prognosis in upper gastrointestinal hemorrhage. Am J Gastroenterol. 1998;93(3):336–340. doi: 10.1111/j.1572-0241.1998.00336.x. [DOI] [PubMed] [Google Scholar]
- 16.Vreeburg EM, Terwee CB, Snel P, Rauws EA, Bartelsman JF, Meulen JH, Tytgat GN. Validation of the Rockall risk scoring system in upper gastrointestinal bleeding. Gut. 1999;44(3):331–335. doi: 10.1136/gut.44.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saeed ZA, Ramirez FC, Hepps KS, Cole RA, Graham DY. Prospective validation of the Baylor bleeding score for predicting the likelihood of rebleeding after endoscopic hemostasis of peptic ulcers. Gastrointest Endosc. 1995;41(6):561–565. doi: 10.1016/S0016-5107(95)70191-5. [DOI] [PubMed] [Google Scholar]
- 18.Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38(3):316–321. doi: 10.1136/gut.38.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaikitamnuaychok R, Patumanond J. Clinical risk characteristics of upper gastrointestinal hemorrhage severity: a multivariable risk analysis. Gastroenterol Res. 2012;5(4):149–155. doi: 10.4021/gr463w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Committee on Trauma, American College of Surgeons. ATLS: Advanced trauma life support program for doctors. 8th ed. Chicago: American College of Surgeons; 2008. [Google Scholar]
- 21.Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356(9238):1318–1321. doi: 10.1016/S0140-6736(00)02816-6. [DOI] [PubMed] [Google Scholar]
- 22.Stanley AJ, Ashley D, Dalton HR, Mowat C, Gaya DR, Thompson E, Warshow U. et al. Outpatient management of patients with low-risk upper-gastrointestinal haemorrhage: multicentre validation and prospective evaluation. Lancet. 2009;373(9657):42–47. doi: 10.1016/S0140-6736(08)61769-9. [DOI] [PubMed] [Google Scholar]
- 23.Kollef MH, O'Brien JD, Zuckerman GR, Shannon W. BLEED: a classification tool to predict outcomes in patients with acute upper and lower gastrointestinal hemorrhage. Crit Care Med. 1997;25(7):1125–1132. doi: 10.1097/00003246-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Saeed ZA, Winchester CB, Michaletz PA, Woods KL, Graham DY. A scoring system to predict rebleeding after endoscopic therapy of nonvariceal upper gastrointestinal hemorrhage, with a comparison of heat probe and ethanol injection. Am J Gastroenterol. 1993;88(11):1842–1849. [PubMed] [Google Scholar]
- 25.Hay JA, Lyubashevsky E, Elashoff J, Maldonado L, Weingarten SR, Ellrodt AG. Upper gastrointestinal hemorrhage clinical—guideline determining the optimal hospital length of stay. Am J Med. 1996;100(3):313–322. doi: 10.1016/S0002-9343(97)89490-9. [DOI] [PubMed] [Google Scholar]
- 26.Atkinson RJ, Hurlstone DP. Usefulness of prognostic indices in upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22(2):233–242. doi: 10.1016/j.bpg.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Enns RA, Gagnon YM, Barkun AN, Armstrong D, Gregor JC, Fedorak RN. Validation of the Rockall scoring system for outcomes from non-variceal upper gastrointestinal bleeding in a Canadian setting. World J Gastroenterol. 2006;12(48):7779–7785. doi: 10.3748/wjg.v12.i48.7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen IC, Hung MS, Chiu TF, Chen JC, Hsiao CT. Risk scoring systems to predict need for clinical intervention for patients with nonvariceal upper gastrointestinal tract bleeding. Am J Emerg Med. 2007;25(7):774–779. doi: 10.1016/j.ajem.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 29.Tham TC, James C, Kelly M. Predicting outcome of acute non-variceal upper gastrointestinal haemorrhage without endoscopy using the clinical Rockall Score. Postgrad Med J. 2006;82(973):757–759. doi: 10.1136/pmj.2006.048462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masaoka T, Suzuki H, Hori S, Aikawa N, Hibi T. Blatchford scoring system is a useful scoring system for detecting patients with upper gastrointestinal bleeding who do not need endoscopic intervention. J Gastroenterol Hepatol. 2007;22(9):1404–1408. doi: 10.1111/j.1440-1746.2006.04762.x. [DOI] [PubMed] [Google Scholar]