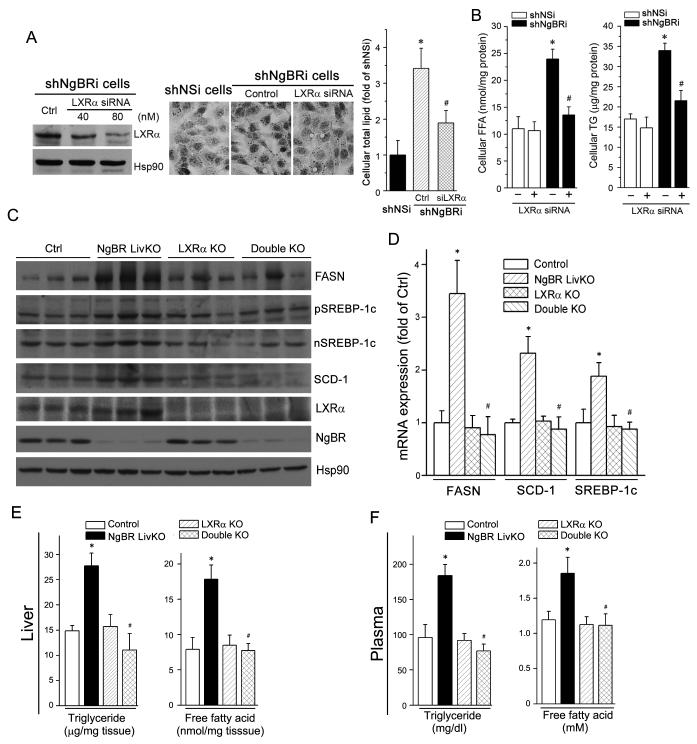
Figure 6. LXRα knockout reduces hepatic lipogenesis in the liver of NgBR LivKO mice.
(A) LXRα knockdown prevents NgBR deficiency-induced lipid accumulation. ShNgBRi cells were transfected with non-silencing siRNA (Ctrl) or LXRα siRNA followed by determination of LXRα expression by western blotting (left panel). Cellular lipid contents were determined by Oil Red O staining (middle panel) and quantitative analysis (right panel). *: P<0.05 vs. shNSi cells; #: P<0.05 vs. shNgBRi cells alone. (B) LXRα knockdown reduces NgBR deficiency-induced accumulation of FFA and TG. shNSi and shNgBRi cells were transfected with LXRα siRNA (80 nM) followed by quantitative analysis of cellular FFA and TG. *: P<0.05 vs. shNSi cells; #: P<0.05 vs. shNgBRi cells alone. (C-F) Lack of LXRα expression attenuates NgBR deficiency-induced lipogenesis in vivo. NgBR and LXRα double knockout (dKO) mice were generated by crossbreeding LXRα KO mice with NgBR LivKO mice. NgBR-floxed (Ctrl), NgBR LivKO, LXRα KO, and dKO female mice at 8 weeks old were fed a Western diet for 4 weeks. Liver and plasma samples were individually collected and used for the following assays. (C) Expression of FASN, (p/n)SREBP-1c, SCD-1, LXRα, and NgBR protein was determined by western blotting; (D) expression of FASN, SCD-1, and SREBP-1c mRNA was determined by real-time RT-PCR and normalized to GAPDH; *: P<0.05 vs. control mice; #: P<0.05 vs. NgBR LivKO mice; (E, F) FFA and TG levels in the liver (E) and plasma (F) were determined with assay kits; *: P<0.05 vs. control mice; #: P<0.05 vs. NgBR LivKO mice (n=6).