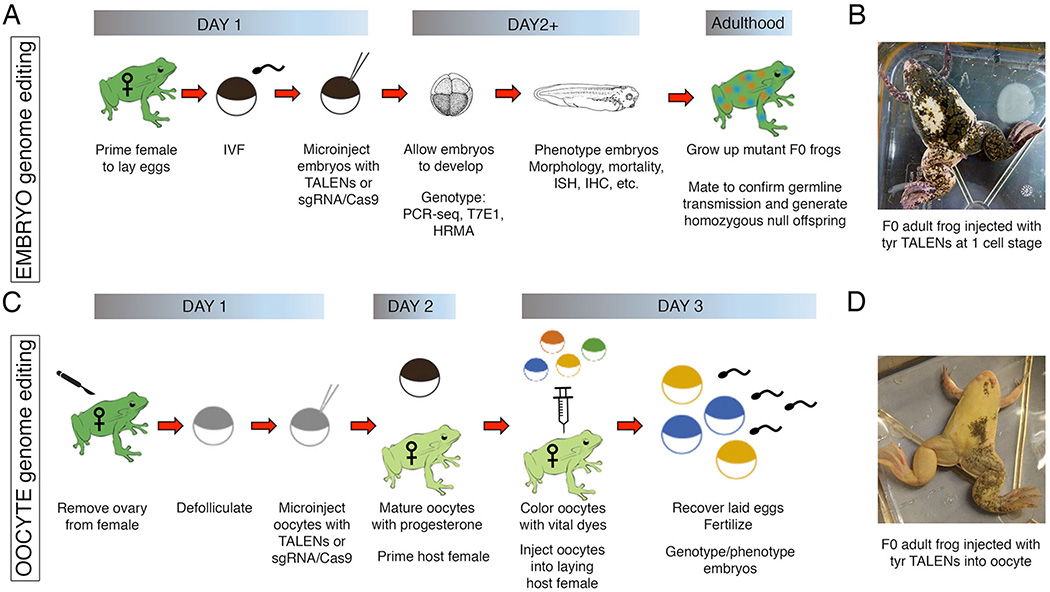
Figure 1. Genome editing using Xenopus embryos or oocytes.
A comparison of the steps required to generate Xenopus mutants using either embryo injection or oocyte-host transfer methods. (A) For the embryo injection method, TALEN or CRISPR-Cas9 capped mRNA and/or protein is microinjected into fertilized embryos at the one-cell stage. The embryos are genotyped to confirm editing efficiency using PCR-sequencing, T7 endonuclease assays, or high-resolution melt analysis. The F0 mosaic embryos are allowed to develop, and gene function is analyzed using a host of established assays, including in situ hybridization (ISH) and immunohistochemistry (IHC). These embryos, once grown to adulthood, can be tested for germline transmission to generate subsequent mutant lines. (B) An image of a mutant frog generated from embryos injected at the one-cell stage with TALEN mRNAs targeting the tyrosinase gene shows mosaic pigmentation throughout the skin. (C) For the oocyte-host transfer method, stage VI oocytes are surgically removed from an adult female frog, manually defollicated, and microinjected with TALENs or CRISPR-Cas9 capped mRNA and/or protein. The oocytes are then matured using progesterone and colored with vital dyes for visualization; the coloring of oocytes is not necessary if implanted into an albino female. The oocytes are then transferred into pre-primed host females and subsequently laid to incorporate the jelly coat that is essential for in vitro fertilization with sperm. The resulting embryos are genotyped and phenotyped as previously described. (D) An image of a mutant frog generated from oocytes injected with the same TALENs as in panel B, targeting the tyrosinase gene, shows more dramatic levels of albinism than the embryo-injected frog, thereby confirming more efficient mutagenesis.