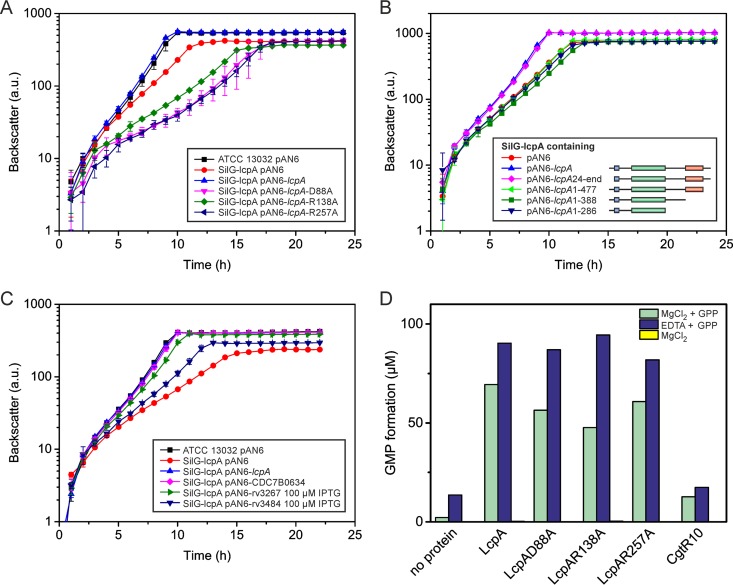
FIG 5.
Complementation experiments and pyrophosphatase assay. (A) Complementation experiment of SilG-lcpA with LcpA variants carrying single mutations of conserved residues in the LCP domain (residues highlighted in Fig. S7 in the supplemental material). (B) Complementation experiment with N- and C-terminally trimmed LcpA variants. (C) Complementation experiment with homologous proteins of the related species C. diphtheriae and M. tuberculosis. Unless indicated otherwise, the growth experiments were performed without induction by IPTG. The mycobacterial proteins were induced to achieve maximal complementation. (D) Pyrophosphatase assay with different LcpAΔTM variants and CgtR10 as a negative control. The proteins were purified from E. coli and incubated in 20 mM Tris-HCl (pH 8.0) with the artificial substrate GPP (1 mM) and either MgCl2 or EDTA at 30°C overnight. The formation of GMP was quantified by LC-MS. No activity was measurable in the absence of the substrate (yellow bars, not visible). One representative result of two independent protein purifications is presented.